Figure 1.
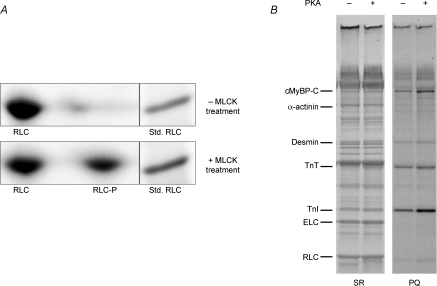
Phosphate incorporation into myofibrillar proteins of skinned murine myocardium following treatment with MLCK or PKA A, SYPRO Ruby-stained two-dimensional SDS–PAGE/IEF gels were used to determine percentage phosphorylation of RLC in skinned myocardium, as shown in these representative gels. The gel in the top panel shows that 2,3-butanedione monoxime (BDM) resulted in little or no detectable phosphorylation of RLC (one spot; RLC), while the gel in the bottom panel shows that subsequent treatment with MLCK resulted in significant phosphorylation of RLC (two spots; RLC and RLC-P). Densitometric scans of the two RLC spots in the gels indicated that ∼40% of total RLC was phosphorylated (RLC-P), consistent with in vivo levels reported previously (Holroyde et al. 1979). When the 2nd dimension was run, a myofibrillar preparation was run in an adjacent lane as a molecular weight standard for RLC. B, SYPRO Ruby- (SR) and Pro-Q Diamond- (PQ) stained gels were used to determine levels of myofibrillar protein phosphorylation with (+) and without (−) PKA treatment, as shown in this representative 10% SDS–PAGE: SR, stained gel for relative abundance of proteins; PQ, stained gel specific for relative abundance of phosphorylated proteins. PKA induced significant incorporation of phosphate in cTnI and cMyBP-C in myocardium. Cardiac troponin T (cTnT), cardiac troponin I (cTnI), myosin essential light chain (ELC) and RLC are also labelled.