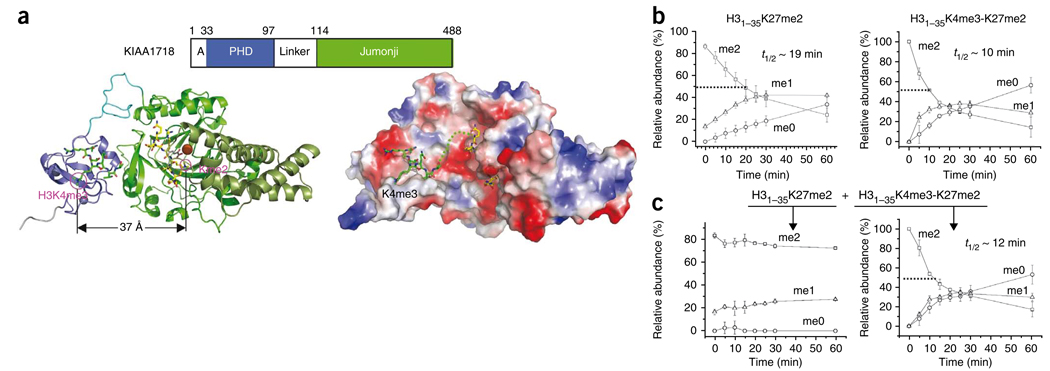
Figure 4.
KIAA1718 selectively demethylates H3K27me2 in the presence of H3K4me3 in cis. (a) A model of KIAA1718 PHD on methylated H3K4 and its linked jumonji active site on a target lysine (left). Surface representation displayed as blue for positive, red for negative and white for neutral (right). The dashed line connects H3K4me3 bound in the aromatic cage and the target lysine in the jumonji domain. (b) The presence of H3K4 methylation in cis enhances KIAA1718 demethylase activities on H3K27me2. (c) When two peptide substrates were mixed in equimolar ratio, H31–35K27me2 (left) and H31–35K4me3-K27me2 (right), KIAA1718 selectively demethylated H31–35 peptides containing both H3K4me3 and H3K27me2 (right).