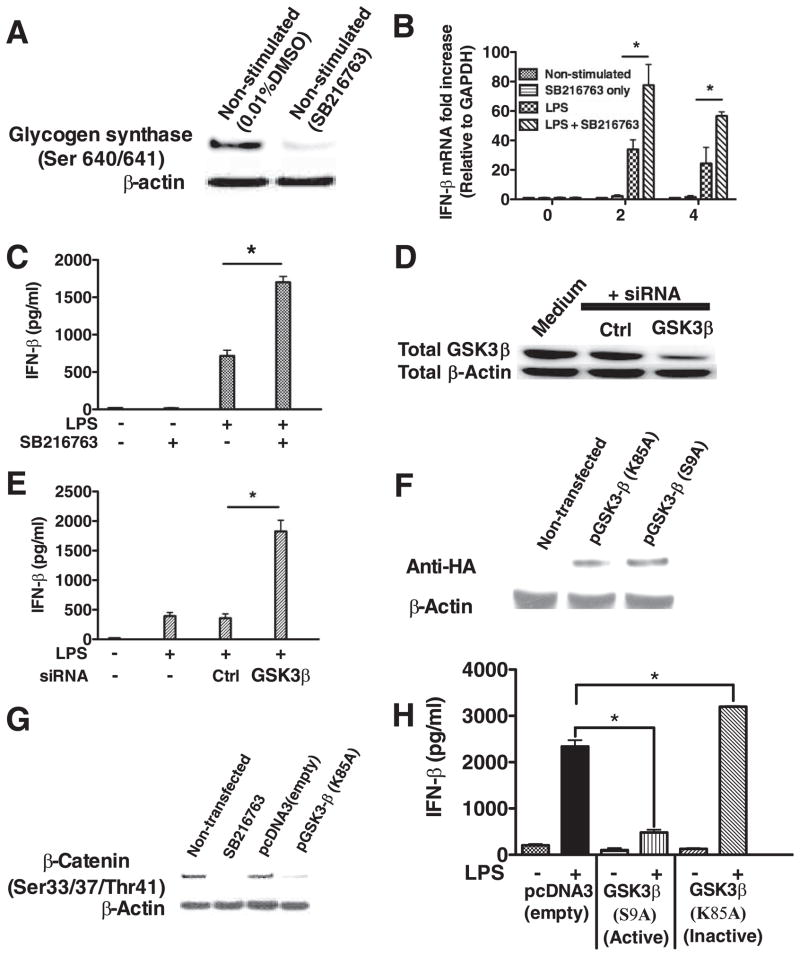
FIGURE 2.
GSK3-β negatively controls IFN-β production by LPS-stimulated macrophages. A, Macrophages treated with the GSK3 inhibitor SB216763 exhibited a loss in the phosphorylation levels of the GSK3-specific substrate glycogen synthase (Ser640/641). Inhibition of GSK3 augmented the mRNA (B) and protein levels (C) of IFN-β produced by TLR4-stimulated macrophages. siRNA-mediated knockdown of GSK3-β protein levels (D) increased the production of IFN-β (E) by LPS-stimulated (1 μg/ml) macrophages. F, HA expression levels in nontransfected macrophages or macrophages transfected with a kinase dead (K85A) or constitutively active (S9A) plasmid encoding GSK3-β. Levels of HA were detected by Western blot 48 h after transfection. G, Expression of the kinase dead (K85A) GSK3 mutant in macrophages inhibited the endogenous phosphorylation of the GSK3-specific substrate β-catenin (Ser33/37/Thr41). H, As compared with empty vector control macrophages stimulated with LPS (1 μg/ml), the constitutively active GSK3-β (S9A) and kinase dead GSK3-β (K85A) negatively and positively, respectively, regulated IFN-β production by macrophages stimulated with LPS (1 μg/ml). *, Statistically significant differences at p < 0.05 between the indicated groups. Results represent the mean ± SD of three separate experiments.