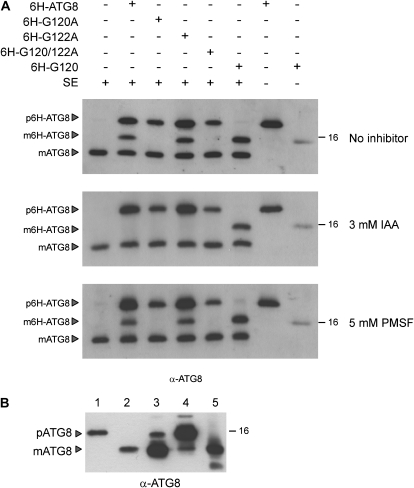
Figure 4.
ATG4 protease activity in cell-free extracts of Chlamydomonas. A, Ten nanograms of the different recombinant ATG8 proteins (CrATG8, G120A, G122A, G120122A, or G120) was incubated with 30 μg of cell-free extracts (SE) of Chlamydomonas during 45 min on ice. When required, cell-free extracts were previously incubated with the corresponding protease inhibitor for 45 min on ice. The reaction was stopped by the addition of loading buffer and boiling at 100°C. Aliquots of the reaction mixtures were analyzed by western blotting with the anti-CrATG8 antibody. Exogenous, recombinant proteins contained the His6 tag and were labeled as 6H to distinguish them from endogenous CrATG8. The precursor (p) and mature (m) forms of CrATG8 are marked with arrowheads. The top panel corresponds to control (without protease inhibitor) assays, while the middle and bottom panels correspond to experiments carried out in the presence of the protease inhibitor 3 mm iodoacetamide or 5 mm PMSF, respectively. B, CrATG8 is constitutively processed in Chlamydomonas. Western-blot analysis of the CrATG8 protein. Lane 1, His tag-free ATG8 purified protein; lane 2, His tag-free G120 purified protein; lane 3, total extract from atg8 yeast cells expressing CrATG8; lane 4, total extract from atg8 yeast cells expressing G120A; lane 5, cell-free extract from Chlamydomonas. The precursor (p) and mature (m) forms of CrATG8 are marked with arrowheads. A molecular mass marker (kD) is indicated on the right.