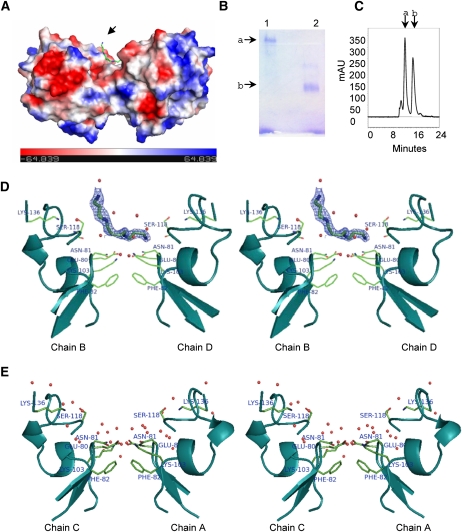
Figure 2.
LS-24 dimer and spermine binding. A, Surface topology of LS-24 dimer along with bound spermine molecule (indicated by an arrow), showing surface charge distribution. Spermine is shown as stick. B, Native gel profile showing the oligomeric state of LS-24. Bands a and b correspond to dimer and monomer, respectively. Lanes 1 and 2 have been loaded with native and heat denatured proteins, respectively. C, Size-exclusion chromatogram of LS-24. Peaks a and b correspond to dimeric and monomeric forms of LS-24, respectively. mAU, Milli absorbance units. D, Stereo view showing interactions of bound spermine at the dimer interface. Bias removed Fo-Fc map highlighting the bound spermine molecule at 1.5 σ cutoff. E, Stereo view showing the interactions at dimer interface without spermine, in another dimer in the asymmetric unit. Chains A, B, C, and D refer to four monomers in the asymmetric unit organized into two dimers, A:C and B:D.