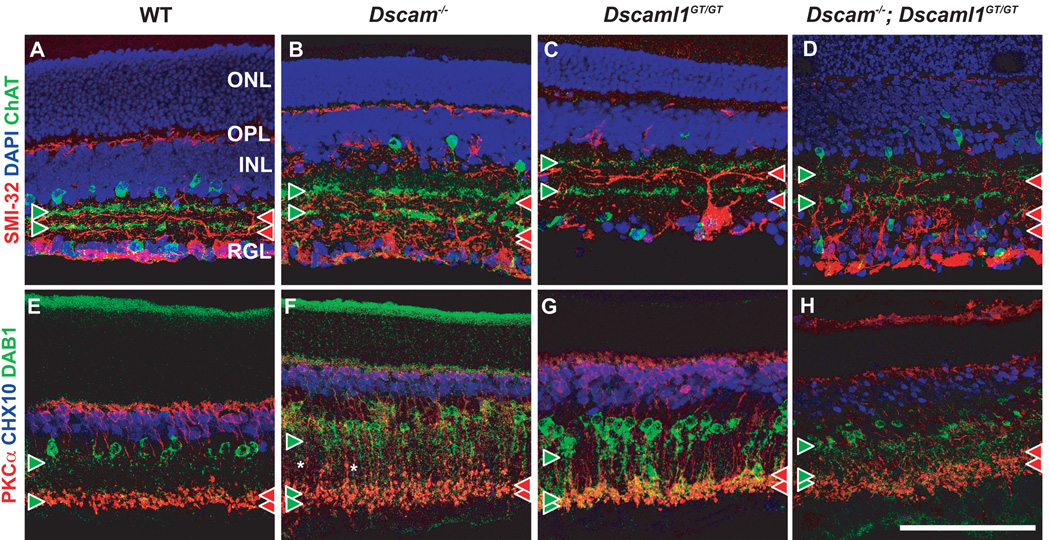
Figure 6. Synaptic lamination is preserved in the Dscam or Dscaml1 null retina.
A–D, Retina sections were stained with antibodies to ChAT, to detect cholinergic Starburst amacrine cells, and SMI-32, to detect alpha RGCs. The location of ON and OFF cholinergic bands are used to demarcate synaptic layers S2 and S4. Paired cholinergic bands (green arrowheads) were observed in wild type and mutant retinas, although their lamination was not as compact in the Dscam−/−; Dscaml1−/− retina. A small number of cholinergic amacrine cell neurites were laminated in an ectopic plexiform layer within the INL of the Dscam−/−; Dscaml1−/− retina. Alpha RGCs stratify dendrites on the RGL proximal side of cholinergic bands (read arrow heads). Stratification proximal to cholinergic bands was observed in all genotypes, with dendrite fascicles observed in the ON portion of the Dscam−/− and Dscam−/−; Dscaml1−/− retina. E–H, Retina sections were stained with antibodies to CHX10, to label bipolar cells, PKCa, to label RBCs and DAB1, to label AII amacrine cells. The axon terminals of RBCs were laminated in S4-S5 of all genotypes. Varicosities were observed in RBC axons in the Dscam−/− and Dscam−/−; Dscaml1−/− retina. AII neurites were stratified in S1/S2 and S4/S5 of all genotypes and also colaminated with the axonal terminals of RBCs in all genotypes. AII amacrine cells were observed to stratify neurites in an ectopic plexiform layer within the INL and to also project neurites past RBC terminals, towards the RGL in the Dscam−/−; Dscaml1−/− retina. The scale bar in (H) is equivalent to 134.6 mm for all panels except D, and H, in which it is equivalent to 123 mm.