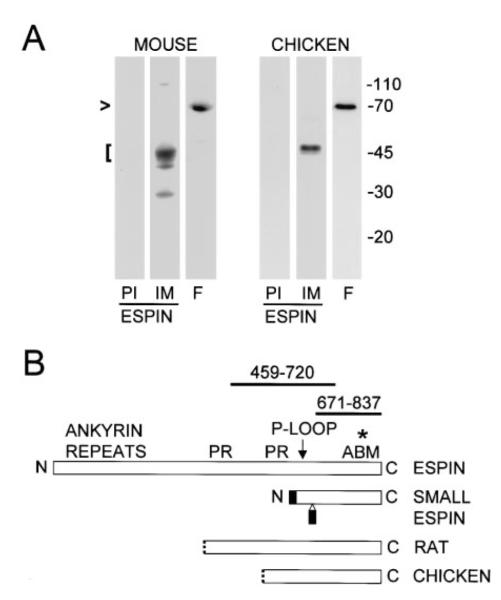
Figure 3. Detection of Inner Ear Espin Proteins and cDNAs by Western Blotting and Library Screening.
(A) Western blots of SDS extracts of mouse temporal bone homogenate and isolated chicken cochlear sensory epithelium showing that the affinity purified espin antibody reacts most strongly with proteins of ~45-50 kD (bracket at left) (PI, preimmune IgG control; IM, affinity purifed espin antibody). Fimbrin (lanes labeled F) remains largely intact at ~68-70 kD in these specimens (arrowhead).
(B) Stick-figure diagram highlighting the relationships between rat espin, rat small espin and the peptides encoded by the longest espin cDNAs obtained from rat and chicken cochlear cDNA libraries (PR, proline-rich peptide; ABM, 116-amino shared C-terminal actin-bundling module). The N-termini of the peptides encoded by the cochlear cDNAs are shown as dotted lines to signify that the cDNAs either are (chicken) or may be (rat) partial at their 5′ ends. None of the cochlear espin cDNAs encoded the two small peptides (shaded) that are unique to small espin (Bartles et al., 1998; Chen et al., 1999). The affinity purified antibodies used in this study are directed against the 167-residue shared C-terminal peptide of rat espin (amino acids 671-837) or a 262-residue peptide from a more central region of rat espin (amino acids 459-720) (thick lines above stick-figure of rat espin). The asterisk designates the position of the point mutation detected in jerker mice, which elicits a frameshift after R808 of mouse espin.