Abstract
Aquaporins form water channels that play major roles in a variety of physiological processes so that altered expression or function may underlie pathological conditions. In order to identify compounds that modulate aquaporin function, we have implemented a functional assay based on rapid measurement of osmotically induced cell volume changes to screen several libraries of diverse drugs. The time course of fluorescence changes in calcein-loaded cells was analyzed during an osmotic challenge using a 96-multiwell fluorescence plate reader. This system was validated using astrocyte primary cultures and fibroblasts that strongly express endogenous AQP4 and AQP1 proteins, respectively, as well as AQP4-transfected cells. We screened 3575 compounds, including 418 FDA-approved and commercially available drugs, for their effect on AQP-mediated water transport. Primary screening yielded 10 compounds that affected water transport activity in both astrocytes and AQP4-transfected cells and 42 compounds that altered cell volume regulation in astrocytes. Selected drugs were then analyzed on AQP1-expressing erythrocytes and AQP4-expressing membrane vesicles by stopped-flow light scattering. Four molecules of the National Cancer Institute's chemical library (NSC164914, NSC670229, NSC168597, NSC301460) were identified that differentially affected both AQP4 and AQP1 mediated water transport, with EC50 values between 20 and 50 μM. This fluorescence microplate reader-based assay may, thus, provide a platform for high-throughput screening which, when coupled to a secondary evaluation to confirm target specificity, should allow discovery of AQP-specific compounds for novel therapeutic strategies in the treatment of water balance disorders.
The discovery of water channel proteins, termed aquaporins (AQPs), has revealed the molecular basis for understanding fluid absorption and secretion through cell membranes in response to even very small osmotic gradients created by solute movement.1–3 Tissue distribution and regulation studies have provided indirect evidence for the involvement of AQPs in major organ functions and in a number of physiological processes. Therefore, malfunctions of channels formed by these proteins may lead to water balance alterations both in epithelial and nonepithelial cells and play critical roles in numerous diseases including glaucoma, brain edema, stroke, tumor growth and spread, congestive heart failure, obesity, and infections.4–8 Phenotype analysis of transgenic mice in which AQPs are knocked out or mutated have provided new insights regarding the role of AQPs in both physiological and pathophysiological conditions9 and have suggested that pharmacological modulation of AQP expression and function may be clinically important. The identification of AQP-selective molecular modulators are, thus, predicted to serve as new tools to analyze AQP function and as potential therapeutic alternatives to current approaches for the treatment of human diseases involving dys-regulation of water balance.10
To date, no AQP modulators that are suitable candidates for clinical development have been discovered. Sulfhydryl-reactive compounds such as mercury and gold have been shown to inhibit some AQPs,11 but these metal ions are nonspecific and extremely toxic to living cells, and their effects are not reversible. Some candidate blockers, including quaternary ammonium compounds,12–14 arylsulfonamide-based carbonic anhydrase inhibitors,15–17 and certain antiepileptics18 have been proposed as putative selective modulators of AQP1 and AQP4, but subsequent measurements in multiple water transport systems did not confirm these data.19–21 In addition, the initial conclusion that DMSO slows AQP-facilitated osmotic water equilibration19,22 has been considered as an apparent inhibition resulting from an osmotic artifact rather than “bona fide” inhibition.
The purpose of our study was to implement a rapid fluorescence-based assay of cell volume changes in a multiwell format to screen several chemically diverse small compound libraries for potential modulators of AQP-mediated water transport. Cells examined included primary cultures of mouse astrocytes (which endogenously express AQP4) and a mouse fibroblast cell line (2497 cells, which express AQP1) as well as an AQP-deficient astrocytoma cell line (TNC1 cells) following stable transfection with AQP4. Chemical compounds identified in the primary screen were subjected to a secondary evaluation to eliminate false positive hits using stopped-flow light scattering applied to plasma membrane vesicles obtained from AQP4-transfected TNC1 cells and rat erythrocytes (expressing abundant AQP1 protein). The results presented here indicate the potential utility of a screening method based on rapid fluorescence measurement of cell volume changes in a multiwell plate format for the discovery of lead compounds that may be useful reagents for experimentally modulating water channel function and may lead to novel therapeutic approaches to disorders of water homeostasis.
METHODS
Astrocyte Primary Cultures
Primary mouse astrocytes were prepared from the cerebral cortices of newborn pups and cultured for 3 to 4 weeks as described.23 Briefly, brains were carefully cleaned of meninges and blood vessels. Neocortical tissues were then dissected away and incubated in Dulbecco's modified Eagle's medium (DMEM) plus 0.25% trypsin. Dissociated cells were centrifuged, resuspended in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin, and incubated at 37 °C in a 5% CO2 atmosphere with a medium change twice a week (all products were purchased from Gibco-Invitrogen, Milan, Italy, or Carlsbad, CA). Immunocytochemistry showed that 98% of the cells stained positively for the astrocytic marker, glial fibrillary acidic protein (GFAP).
Cell Lines and Erythrocytes
Cell cultures were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen Life Technologies) supplemented with 10% fetal bovine serum (Euroclone), 100 U/mL penicillin, 100 μg/mL streptomycin. Cells were grown at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The TNC1 cell line, established from primary cultures of type 1 astrocytes from brain diencephalon tissue of 1 day old rats,24,25 was purchased from American Tissue Culture Collection (ATCC, Rockville, MD). Mouse fibroblast 2497 cells expressing endogenous AQP1 in culture were from LGC Standards (UK).
Erythrocytes were obtained from whole blood samples drawn from Wistar rats in sodium heparin buffer and washed three times at 800g at 4 °C for 10 min in phosphate-buffered saline to remove plasma and the cellular buffy coat. Red blood cells were stored at 4 °C and used within 24 h of collection.19
Transfection of the TNC1 Cell Line
To generate TNC1 cells stably transfected with AQP4, pcDNA3 vector (Invitrogen) containing the cDNA sequence for the M23 (aa23–323) isoform of rat AQP4 was used. Cells were transfected using Lipofectamine Plus Reagent (Gibco BRL, Grand Island, NY) according to the manufacturer's directions. Briefly, cells were grown on 35 mm diameter dishes to 90–95% confluency the day of transfection, washed twice with DMEM without antibiotics, and cotransfected with 3 μg of AQP4 expression plasmid and 1 μg of pBabe vector conferring puromycin resistance. The cells were incubated for 6 h, after which the plasmids/Lipofectamine Plus Reagent mix was replaced with standard culture medium. For stable clones, AQP4-expressing cells were initially selected for two weeks in medium with 5 μg/mL puromycin and maintained thereafter in 2 μg/mL puromycin.
SDS-PAGE and Western Blot Analysis
Protein samples were separated on 12% NuPAGE gels (Invitrogen Life Technologies) and electrophoretically transferred to PVDF membranes (Millipore, Bedford, MA). Immunoblotting was performed as previously described.26,27 Briefly, membranes were probed overnight with the primary antibodies against AQP4 (goat anti-AQP4 polyclonal antibody (C-19) 1:1000; Santa Cruz Biotechnology) or AQP1 (rabbit anti-AQP1 polyclonal antibody (L-19) 1:500; Santa Cruz Biotechnology), as appropriate, washed with blocking solution, and incubated for 1 h with HRP-conjugated donkey antigoat IgG or antirabbit IgG secondary antibody (1:5000; Santa Cruz Biotechnology). Reactive proteins were revealed with an enhanced chemiluminescent detection system (ECL-Plus; GE Healthcare) and visualized on a Versadoc imaging system (Biorad Laboratories).
Immunocytochemistry
Cultured cells on glass coverslips were fixed in 4% paraformaldehyde, washed in phosphate buffered saline (PBS), and permeabilized with 0.3% Triton X-100 in PBS. After blocking with 0.1% gelatin in PBS, cells were incubated with appropriate primary antibodies for 1 h at RT. The following primary antibodies were used, goat anti-AQP4 polyclonal antibody (1:400; (C-19) Santa Cruz Biotechnology) and rabbit anti-AQP1 polyclonal antibody (1:400; L-19) Santa Cruz Biotechnology). After washing in PBS, cells were incubated for 30 min with Alexa 488 and/or Alexa 594 conjugated secondary antibodies (Molecular Probes, Eugene, OR). Coverslips were finally mounted in PBS–glycerol (1:1) containing 1% n-propylgallate, pH 8.0, and examined with a photomicroscope equipped for epifluorescence (DMRXA, Leica, Heidelberg GmbH, Mannheim, Germany). Digital images were obtained with a DMX 1200 camera (Nikon, Tokyo, Japan).
Screening Procedure
Screening was carried out using a benchtop fluorescence platereader with integrated liquid handling (FlexStation II, Molecular Devices, MDS Analytical Technologies, USA) equipped to perform functional cellular assays and to analyze real time fluorescence kinetic data in the 96-well format. The instrument consists of an incubated cabinet with fluorometer and integrated 96 channel pipettor which is able to transfer compounds from one microplate to the assay plate, allowing rapid kinetic assays. Data acquisition was performed by SoftMax Pro software, and the data were analyzed with Prism (Graphpad) software.
Cells were plated in 96-well black-walled microplates (Corning-Costar Corp., Corning, New York) at a density of 12 000 cells per well. Water permeability assays were done at 24–48 h after plating at which time cells were 80–85% confluent. Our screening strategy was based on the calcein quenching method reported by Hamman et al.28 Cells were washed with PBS and incubated at 37 °C for 45 min with 10 μM of membrane permeable calcein-AM (Molecular Probes, Eugene, OR), which is trapped intracellularly following cleavage by esterases. The resulting membrane-impermeant calcein fluorophore exhibits strong concentration dependent self-quenching so that measured changes in fluorescence were directly proportional to changes in cell volume.29 After rinsing in 50 μL PBS, test compounds of a specific drug set were added to individual wells to give 20 μM final drug concentration and 1% final DMSO concentration. After a 15 min incubation at 37 °C, the 96-well plate was transferred into the plate-reader for the fluorescence assay. Fluorescence was excited at 490 nm and detected at 520 nm using dual monochromators. Time course fluorescence data following mixing of cells with hyper- or isosmotic solutions were recorded over a 90 s period. We assayed each well for water transport by recording fluorescence continuously for 15 s (baseline), then for 35 s after rapid automated addition of hyperosmolar PBS (7.5 μL of 0.5 M NaCl), and for 40 s after automated addition of distilled water to restore the isosmotic condition. To optimize the number of data points, we set the instrument to read out sequentially the top half of the plate and the bottom one. Each time point was, thus, obtained every 0.5 s. The time constant of cell shrinkage due to the hypertonic stimulus was obtained by fitting the data with an exponential function.
To assess the quality of the screening assay,30,31 we computed the statistical Z′ factor using data from astrocyte assay plates containing negative controls (in the absence of inhibitor) and positive controls (in the presence of inhibitor) as defined by Z′ = 1.0 – (3.0 × (SDpos + SDneg)/(Apos – Aneg), where SD and A represent the standard deviation and mean time constant values for positive controls (pos) and negative controls (neg).
Compounds for Medium-Throughput Screening
Primary screening included a library of 418 FDA-approved compounds kindly provided by Dr. David Lawrence (see Acknowledgments) that have been characterized for their safety profile in humans and their mechanism of action and biochemical targets (see supplementary Table 1 in the Supporting Information). In addition, we used a collection of 3 157 drug-like compounds provided by the National Cancer Institute (NCI) in frozen bar-coded 96-well plates, each well containing 20 μL of 1 or 10 mM stock solution in DMSO with plate map accession numbers (see supplementary Table 2 in the Supporting Information).
The NCI plated sets used for screening were challenge, diversity, mechanistic, and natural products sets (see supplementary Table 2 in the Supporting Information) (http://dtp.nci.nih.gov/branches/dscb/repo_open.html). The challenge set includes 57 diverse compounds with unknown mechanisms of cell killing. The structural diversity set includes 1990 compounds selected for effects on cell proliferation in the NCI cell line screen. The mechanistic set consists of 879 compounds selected from ~40 000 compounds that have been tested in a panel of 60 human cancer cell lines and represents a diverse range of growth inhibition patterns. The natural products set contains 235 structurally diverse natural products. The sets include many known bioactive compounds as well as diverse synthetic and natural products with unknown biochemical mechanisms. These compounds were screened at final concentrations of 10–50 μM. Plates (96 well) containing one compound per well (each 2 mM) were prepared for screening and stored frozen in DMSO.
Preparation of Plasma Membrane Vesicles
Membrane vesicles from TNC1-AQP4 transfected cells were prepared as previously described with minor modifications.32 Cells from several 150 mm diameter plastic dishes were washed two times with Ca2+/Mg2+-free PBS, scraped in homogenizing buffer (HB, 300 mM sucrose, 1 mM EDTA, 1 mM PMSF, 1 ug/mL pepstatin A, 1 ug/mL leupeptin, 10 mM Tris–HCl, pH 7.2) and homogenized by five strokes with a Potter-Elvehjem homogenizer. The homogenate was spun at 4000g for 15 min, and the supernatant was centrifuged at 17 000g for 45 min to obtain a fraction enriched in plasma membranes. Protein concentration was measured with BCA Protein Assay Kit (Biorad, Rockford, IL).
Stopped-Flow Measurements of Water Permeability in TNC1-AQP4 Membrane Vesicles and Erythrocytes
Water permeability measurements were done by a light scattering method using a stopped-flow SFM-20 instrument (Biologic, Science Instruments, Claix, France).33,34 Membrane vesicles from TNC1-AQP4 transfected cells were resuspended in 0.5 mg of protein/mL in 50 mM sucrose, 10 mM Tris–HCl, pH 7.4 and subjected to a 250 mOsm inwardly directed sucrose gradient. The kinetics of decreasing vesicle volume were measured from the time course of 90° intensity changes at 530 nm wavelength. Time constant (τ, s) was computed from the light scattered signal, and the vesicle size was determined by quasi-elastic light scattering using a Coulter model N5 multiangle particle size analyzer (Beckman-Coulter Inc., Palo Alto, CA).
Red blood cells were obtained as described above, diluted to a hematocrit of 1% in PBS and mixed within 1 ms with an equal volume of hyperosmolar PBS to give a 250 mOsm inwardly directed osmotic gradient. The time course of osmotic cell shrinkage was recorded at 600 nm wavelength. Measurements were done at low temperature (12 °C) in order to minimize the diffusive temperature-dependent water transport through the lipid phase of the cell membrane. To verify the specificity of the drugs detected as potential hits in the multiwell fluorescence screen, membrane vesicles or red blood cells were incubated with test compounds for 15 min before stopped-flow measurements. Solution osmolalities were measured using a vapor pressure osmometer (Wescor, USA).
Concentration-Inhibition Analysis
Dose–response curves were fitted using Prism (Graphpad) software to the following equation:
where y is the relative rate constant for shrinking (max 0 = 1), [Inh] is the concentration of inhibitor, EC50 is the concentration for 50% inhibition, and n the Hill slope.
RESULTS
Development of the Functional Assay
Assay development for compound screening was first performed on primary mouse astrocyte cultures and the 2497 fibroblast cell line that strongly express endogenous AQP4 and AQP1 proteins, respectively. Immunofluorescence analysis performed on cells grown on coverslips confirmed the corresponding expression of AQP4 in mouse astrocytes and AQP1 in fibroblasts (Figure 1A). Western blot results obtained using membranes prepared from mouse astrocytes and the fibroblast cell line and probed, respectively, with anti-AQP4 and anti-AQP1 antibodies are shown in Figure 1B. A major band of 30 kDa together with a less intense 32 kDa protein band was detected with AQP4 antibodies in the lane containing proteins from the membrane fractions of primary cultured astrocytes. These two bands correspond to M23 and M1 splice variants of the AQP4 protein.35 Membrane fractions from fibroblasts, probed with AQP1 antibodies, revealed a 28 kDa band corresponding to the molecular size of the AQP1 protein.
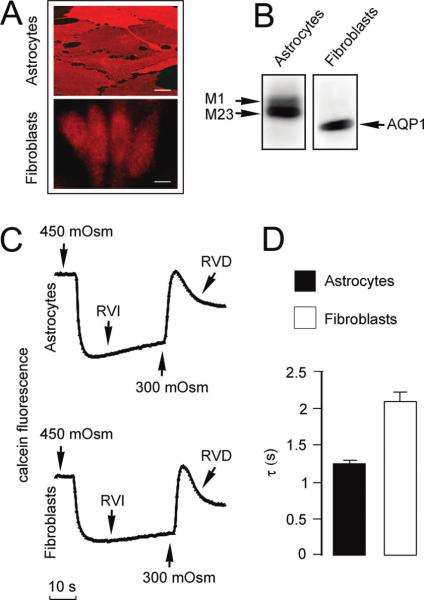
Figure 1.
Rapid osmotic responses of mouse astrocyte cultures and the fibroblast 2497 cell line expressing endogenous AQP1 and AQP4 proteins, respectively. (A) Immunofluorescence analysis of AQP4 protein in primary mouse astrocyte culture and AQP1 protein in the fibroblast cell line. Bar = 15 μm. (B) Immunoblot analysis of plasma membranes prepared from mouse astrocytes and fibroblasts and probed, respectively, with anti-AQP4 and anti-AQP1 antibodies. Note the presence of two AQP4 isoforms, termed M1 and M23. (C) Representative recordings of reversible changes in calcein fluorescence observed in response to hyperosmotic shock in mouse astrocytes and in a fibroblast cell line. Hypertonicity was obtained by adding NaCl to PBS to reach 450 mOsm final osmolarity (change of solution at the first arrow), and then, it brought back to an isosmotic solution by addition of water (upward arrow). Fluorescence was measured as a function of time and was directly related to the cell volume. In addition to the rapid phases in the responses, note the typical RVI (regulatory volume increase) and the RVD (regulatory volume decrease) present in responses of both astrocytes and fibroblasts. (D) Average time constant (τ) values for initial cell shrinkage observed in experiments as in (C) calculated from exponential fittings of the data (mean ± SE, ten measurements per cell line).
In order to screen libraries of druglike compounds for modulation of AQP4 function, we implemented a functional assay based upon calcein fluorescence quenching, a well-established method for measurement of rapid osmosis in monolayers of cultured cells.28,29 Cultured cells in the 96-well format were loaded with calcein-AM, a membrane-permeable calcein derivative that is cleaved intracellularly by esterases to become trapped in the cytoplasm. The functional assay of AQP-expressing cells was performed in a fluorimetric plate reader as described in the Methods. The volumes, rates, and hardware for solution additions were optimized to avoid artifactual fluorescence signals during continuous recordings from individual wells. Cells were rinsed in 50 μL of isosmolar PBS, and fluid transfer volumes to be dispensed from the source to each individual well were set to ensure that the tip of the multichannel pipettor was below the surface of the liquid at the end of the transfer, thus minimizing the possibility that undispersed drops remained in the tips. We also monitored the mixing time in a single well of the assay microplate by choosing various rate values at which the fluid is dispensed into the well among those available in the SoftMax Pro software. In a 96-well plate, flow rate may be set from 26 to 125 μL/s by increments of 26 μL. The accurate recording of the time course of cell volume changes was satisfied by the rapid mixing time (~400 ms) obtained when the flow rate of the added solution was 47–62 μL/s, without causing detachment of cells and disrupting the integrity of the cell layer as assessed by microscopic analysis of the calcein-loaded cells before and after the experiment. The solution mixing time was measured by detecting the absorbance change upon mixing colorless and dye-containing aqueous solutions in a single well of the assay plate. The short duration of the solution mixing provides adequate temporal resolution to compute cell shrinkage kinetics in response to a hypertonic stimulation and minimizes ambiguity in analysis of the cell kinetics. During the optimization of the assay, we determined that comparable assay results were obtained in wells plated with 1000 to 15 000 cells and within 2 days after seeding.
Figure 1C illustrates representative time courses of changes in the fluorescence signal following cell shrinkage in response to solution exchange between isotonic and hypertonic saline in both astrocytes and fibroblasts. Calcein fluorescence was clearly dependent on perfusate osmolalities.
The signal decreased upon robotic addition of hyperosmolar PBS (450 mOsm final concentration) due to cell shrinkage and quenching of calcein emitted fluorescence. In contrast, after return to iso-osmolarity, the signal increased as a consequence of cell swelling and increasing of calcein fluorescence intensity. Cell volume changes were reversible, and the recorded data were highly reproducible from well to well.
Both types of cultured cells showed a fast osmotic response, reaching an apparent steady state in less than 5 s after the onset of an osmotic challenge with an apparent volume shrinkage of about 30% compared with an isotonic condition. In addition to the rapid phases in the responses, cells exhibited remarkable volume regulation after the osmotic shock, resulting in a regulatory volume increase (RVI) after the shrinkage period and in a regulatory volume decrease (RVD) when the hypertonic buffer was changed to isotonic buffer. These processes indicated that cell swelling and shrinkage involve the movement of ions and other osmolytes across the cell membrane.
The initial region of the fluorescence curve after the hypertonic shock was fitted with a single exponential function, and its time constant was used as a quantitative measure of the rate of cell shrinkage. This value is independent of the absolute values of fluorescence intensity at the beginning of the cell shrinkage, as observed in wells plated with a range of cell densities reported above. The average time constant, τ, for the calcein fluorescence response was 1.25 ± 0.04 s (n = 12) for astrocytes and 2.1 ± 0.13 s (n = 20) for fibroblasts (Figure 1D). The cell shrinkage rate, as well as the amplitude of the volume change, was highly reproducible after repeated hypertonic challenges applied in the same well. These data suggest that the osmotic water transport across the cellular membrane is a fully reversible process and that the loss of electrolytes from the cells is not significant.
In order to verify that the osmotic challenge induced by increasing the concentration of Na and Cl ions did not affect activity of transporters and other ion channels, we performed additional experiments where the same hyperosmolarity was obtained with a neutral solute (mannitol). The time constant values measured in this condition were similar to those reported above for NaCl suggesting no significant water movement due to cotransport processes (1.4 ± 0.06 s (n = 5) for astrocytes and 2.5 ± 0.09 s (n = 5) for fibroblasts).
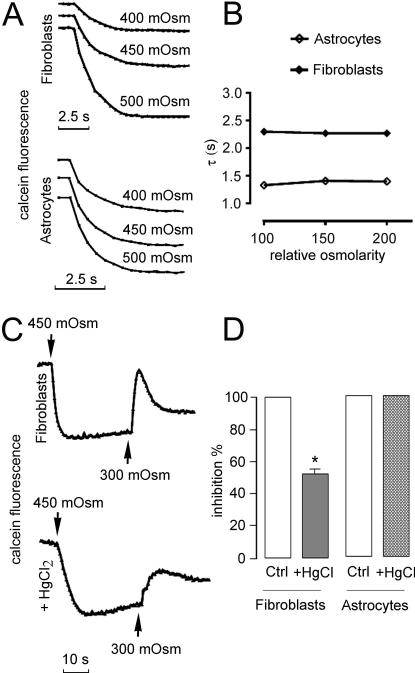
The dependence of the kinetics of changes in fluorescence signal on the magnitude of external osmolality was also examined in astrocytes and fibroblasts. Figure 2A shows a representative calcein quenching time course in which both types of cultured cells were mixed with appropriate volumes of 0.5 M NaCl solution to give the indicated osmolalities. The effect of varying hypertonic osmolarity on the responses of these cells is plotted in Figure 2B. Consistent with a channel-mediated water permeation, the relative signal amplitude was found to be sensitive to the size of the osmotic gradient, whereas the time constant values were largely unaffected.
Figure 2.
Characterization of calcein quenching responses for determination of water transport in fibroblasts and primary mouse astrocytes. (A) Dependence of shrinkage curves on the hypertonic osmolarity in both primary astrocytes and fibroblasts. (B) Graph showing the effect of the osmotic gradient size on the time constants in both astrocytes and fibroblasts. Note that the magnitude of the fluorescence signal is sensitive to the size of the osmotic gradient whereas the shrinkage time constant was unaffected. (C) Representative time course of cell calcein fluorescence changes in response to osmotic challenge in AQP1-expressing fibroblasts (top) under control conditions and after 3 min preincubation with 0.2 mM HgCl2. Bottom panel illustrates the lack of effect of HgCl2 on shrinkage of astrocytes. (D) Average values of time constants (τ), obtained from three sets of experiments as in (C). As predicted, MgCl2 exposure significantly inhibited fibroblast cell shrinkage rate (52.7 ± 3%) (*P < 0.001 compared to control). In contrast, the kinetics of cell shrinkage did not change significantly in cultured astrocytes.
Sulfhydryl-reactive mercurials such as HgCl2 are the best established inhibitors of most aquaporin water channels except for AQP4 and AQP7.23 Figure 2C shows the effect of HgCl2 on water permeability in AQP1-expressing fibroblasts. A significant reduction of the cell shrinkage rate (52.7 ± 3%) was found in fibroblasts after 3 min preincubation with 0.2 mM HgCl2 (Figure 2D). AQP1 inhibition affected also the rate of RVD which is in agreement with several reported actions of mercurials on membrane systems that can potentially influence conductive ion fluxes.36,37 As expected, the time course of cell shrinkage did not change significantly in cultured astrocytes pretreated with HgCl2 (Figure 2C,D).
Transfection of TNC1 Cells with the AQP4 Gene
As astrocyte primary cultures also possess a sustained AQP4-independent water transport pathway through the lipid bilayer,23,29 it is difficult to estimate the effective contribution of AQP4 on the water movement through their plasma membrane and the effect of drugs on the AQP's water permeability. To this end, we screened a number of cell lines (RIN, HeLa, COS-7, Neuro2A, CHO, etc.) for their suitability for AQP activity measurement and drug screening application. We first evaluated the growth on uncoated 96-well plates and the lack of endogenous expression of AQPs. We also analyzed whether the basal osmotic water permeability was low and, thus, likely to be attributable solely to transport through lipid membranes.
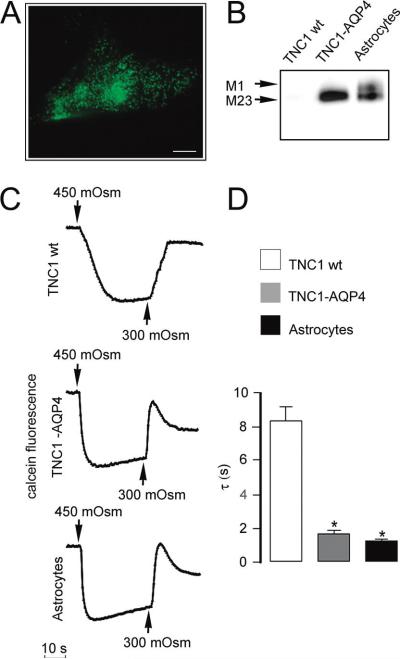
An immortalized astrocyte cell line (TNC1) was selected for its low water permeability (τ = 8.2 ± 0.9 s; n = 20) and no detectable levels of known aquaporins.24,25 The other cell lines had τ values ranging from 2.5 to 4 and, thus, were not suitable for AQP transfection and the functional screening. TNC1 cell growth and adherence do not require coating of the multiwell plates, and adherence was found to be resistant to the washing and solution dispensing procedures. The TNC1 cell line was used to generate clonal cell lines in which AQP4 was stably expressed. Cells were transfected with cDNA corresponding to the open reading frame of M23 isoform of rat AQP4. Selection of transfectants over 2 weeks yielded cell clones that displayed high levels of M23-AQP4 expression, as demonstrated by immunofluorescence analysis (Figure 3A), that revealed a typical dotlike staining pattern that has been shown to correspond to the intramembraneous arrangement of M23-AQP4 in orthogonal arrays of particles (OAPs)38–40 first visualized by freeze-fracture microscopy.38 Accordingly, immunoblot analysis confirmed the positive clones (Figure 3B).
Figure 3.
Characterization of AQP4-transfected TNC1 cells by immunochemical and functional studies. (A) Immunofluorescence image showing the expression of AQP4 protein in the transfected cells. Note the typical punctate staining pattern. No specific staining was detected in nontransfected cells (data not shown). Images were obtained with a 100× objective lens. Bar = 15 μm. (B) Immunoblot analysis of plasma membranes prepared from TNC1 cells stably transfected with the M23 form of AQP4 compared with untransfected TNC1 cells (TNC1 wt) and cultured primary astrocytes as a positive control. (C) Representative calcein signal changes reflecting reversible cell shrinkage after hyperosmotic shock in TNC1 wt cells, AQP4 transfected TNC1 cells, and primary astrocytes. Note the fast osmotic responses and the appearance of strong RVD in transfected cells and in astrocytes and absence of these phenomena in the wt TNC1 cells. (D) Average time constants (τ) for experiments as in (C) calculated from exponential fitting of the data (mean ± SE, five measurements per cell line).
To determine whether AQP4-transfected TNC1 cells expressed functional water channels, we performed water permeability measurements. Time courses of the calcein fluorescence intensity representative for TNC1 wt cells and TNC1 cells transfected with AQP4 compared with those relative to primary culture of astrocytes are shown in Figure 3C. The kinetics of cell shrinkage after hyperosmotic shock were markedly enhanced in AQP4-transfected cells, with a significantly, approximately 5-fold, lower time constant τ value compared to wt-TNC1 cells and consistent with astrocyte primary culture water transport rates (Figure 3C,D). These data suggest that AQP-mediated water transport is the major pathway for the initial shrinkage in the AQP4-expressing cell line. Furthermore, whereas untransfected-TNC1 cells did not show significant RVI or RVD, transfected cells displayed strong RVD and RVI behavior, similar to that found in astrocytes and fibroblasts (Figures 3C and 1C).
Primary Screening of Compound Collections
We applied the fluorescence dilution method to screen chemical libraries for alteration in cell volume responses in a fibroblast cell line, in cultures of primary mouse astrocytes, and in AQP4-transfected TNC1 cells, all cultured in 96-well plates. For primary screening, we used several compound collections from the National Cancer Institute consisting of structurally diverse small molecules that vary in terms of functional groups and charge (see Methods) and a small library of about 400 FDA-approved drugs (obtained from Dr. David Lawrence, see Acknowledgment). Compounds were tested individually by incubating the cells for 15 min at a 20 μM final concentration using the automated plate reader. After the screening, 10 molecules were found to affect water transport activity in both astrocytes and AQP4-TNC1 cells, and 42 molecules altered cell volume regulation in astrocytes. The compounds that affected the time constant of the shrinking curve all belong to the mechanistic set whereas molecules involved in volume regulation mechanism were also present in the challenge and structural sets. Interestingly, we also detected a number of FDA-approved drugs that affected water flux properties in cultured astrocytes.
To evaluate the suitability of the functional assay for hit identification, we calculated the Z′ factor, a simple and dimension-less statistical parameter that provides a useful tool for assay quality assessment.31 To this end, we ran repetitive assays on samples for negative and positive controls such as astrocytes incubated with a representative inhibitor vs control astrocytes. Under our optimized conditions we calculated a Z′ factor of 0.56, a high value that validated the reproducibility of the screening assay.
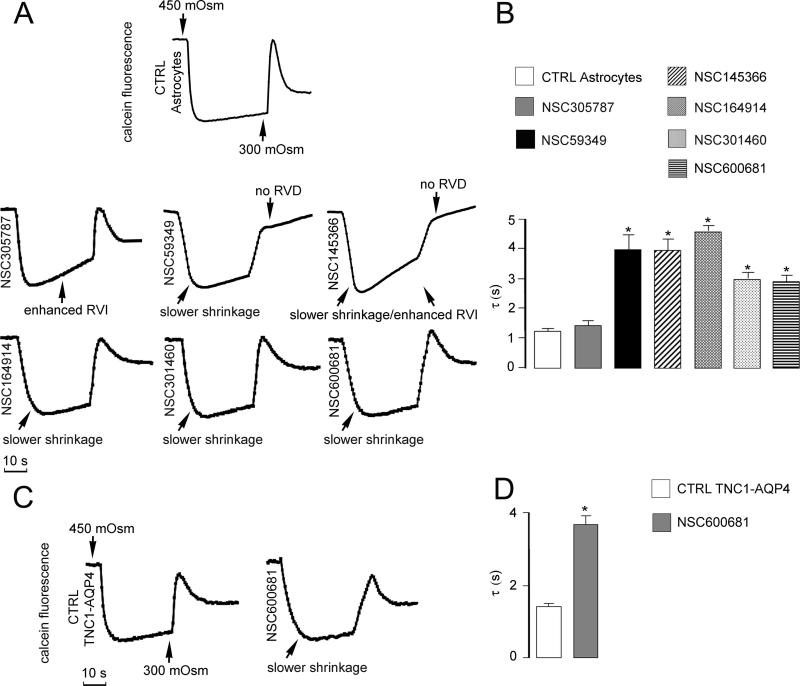
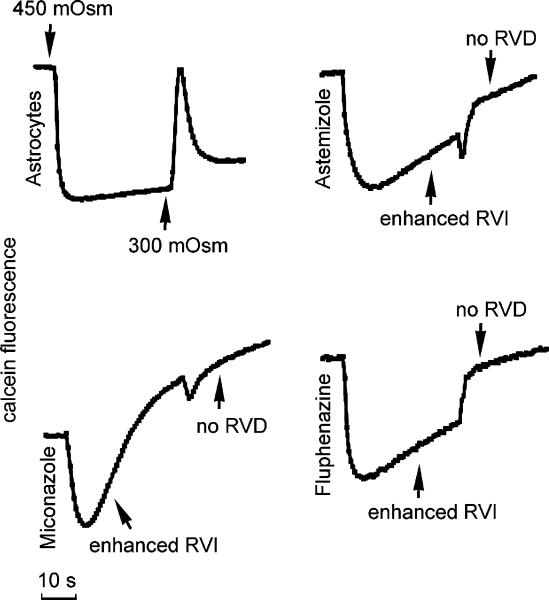
Retesting of hits and their evaluation in untransfected-TNC1 cells was done to reconfirm the activity and to eliminate false positives. Representative curves of the effects produced by some selected hits belonging to the mechanistic diversity set on water flux kinetics of cultured astrocytes (panel A) and AQP4-transfected cells (panel C) are shown in Figure 4. Active compounds produced different and specific patterns of alteration in the water transport rate. In particular, the majority of the compounds, when used at 10–20 μM concentration, affected RVI and RVD, with only a few compounds that were affecting AQP water transport. The bar graph in Figure 4 B summarizes the average time constant values measured in primary astrocytes after exposure to the indicated drugs and in control conditions. A representative effect produced by one of the selected chemicals (NSC600681) tested on AQP4-transfected cells is illustrated in Figure 4C. The AQP4-mediated cell shrinkage time constant was significantly longer after incubation with 20 uM of this compound, reflecting a reduced water transport rate (Figure 4 D). As shown in Figure 5, among the FDA-approved drugs, we identified several molecules that at 20 μM induced significant alteration of cell volume regulation, including astemizole, miconazole, and fluphenazine, whereas other compounds in the same experimental conditions showed no effects.
Figure 4.
Representative effects of some of the tested drugs on water transport properties of cultured mouse astrocytes and AQP4-transfected cells. (A) Different patterns of alteration in water flux kinetics of cultured astrocytes due to a series of drugs of mechanistic and structural sets: NSC305787 enhanced RVI; NSC59349 slowed shrinkage and abolished RVD; NSC145366 enhanced RVI, abolished RVD, and slowed shrinkage; NSC164914 and NSC301460 both affected shrinkage rate. All test compounds were incubated at 20 μM for 15 min before measurements. (B) Average time constants (τ) from five sets of experiments as in (A) calculated from exponential fitting of the data (mean ± SE). (C) Altered water permeability produced by one of the selected chemicals (NSC600681) tested on AQP4-TNC1 cells. (D) Average time constants (τ) from five sets of experiments as in (C) calculated from exponential fitting of the data (mean ± SE). *P < 0.01.
Figure 5.
Representative effect of some FDA-approved compounds on water permeability in mouse astrocytes. Altered cell volume regulation produced by FDA-approved compounds such as fluphenazine, astemizole, and miconazole. All test compounds were incubated at 20 μM for 15 min before measurements. Note that all compounds affected cell volume regulation after an osmotic shock.
Selection of Lead Compounds for Further Development
In order to eliminate molecules that affect calcein fluorescence independently from the AQP-mediated process, a secondary evaluation of hits for compounds that affected water transport in the primary screen was carried out with a stopped-flow technique. In this technique, the intensity of scattered light intensity is used to follow cellular and/or vesicular volume changes. The specificity of the selected compounds were verified by analyzing their effect on plasma membrane vesicles obtained from AQP4-transfected cells and in red blood cells, which endogenously express AQP1.
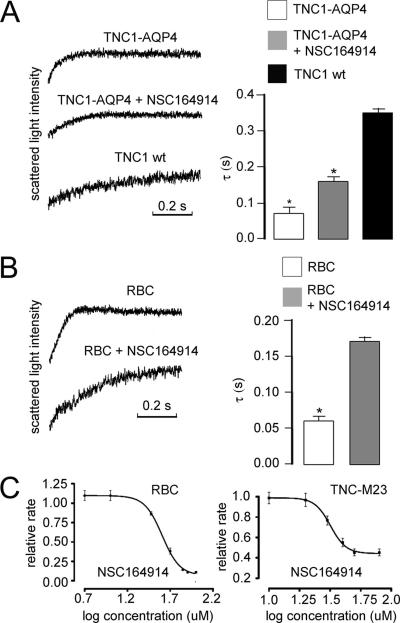
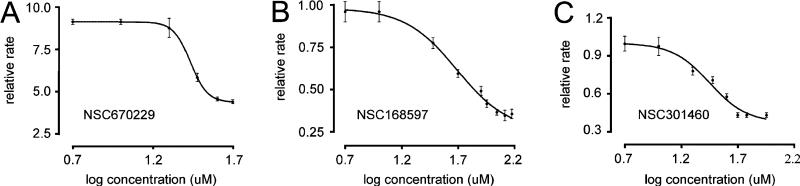
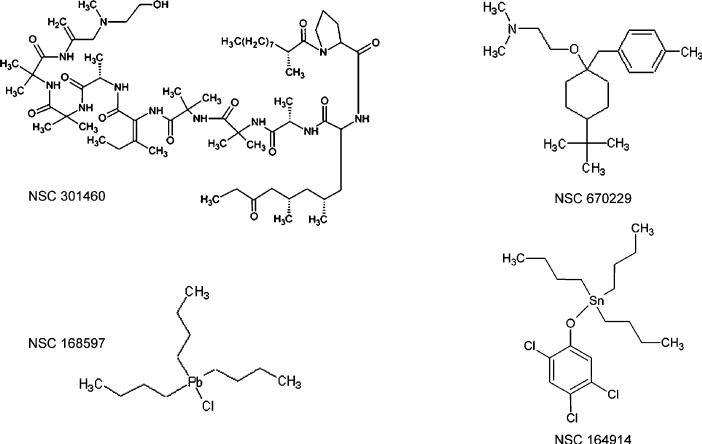
Osmotic water permeability was measured by rapidly mixing the vesicle suspensions with a hyperosmolar solution that causes vesicle shrinkage and increased scattered light intensity. As expected, calculated Pf was much higher in plasma membrane vesicles from AQP4-containing vesicles (0.0120 ± 0.0003 cm/s) than in membrane vesicles from parental cells (0.0020 ± 0.0003 cm/s). Original stopped-flow water transport data for one representative compound (NSC 164914) tested at 20 μM on membrane vesicles from AQP4-expressing cells are shown in Figure 6A. This compound greatly slowed (by 55%) shrinkage kinetics in AQP4-containing vesicles whereas no significant reduction was found in vesicles from TNC1-untransfected cells (data not shown). To determine the functional effect of the compound on other AQPs, we performed stopped-flow experiments on suspensions of red blood cells that express large amounts of AQP1 protein. Figure 6B shows representative light scattering data obtained in rat erythrocytes in control conditions and in the presence of the NSC164914 compound. The compound at 40 μM inhibited the shrinkage rate of native AQP1-expressing erythrocytes by ~64%. To further characterize the observed inhibition of osmotic water flow, plasma membrane vesicles from AQP4-expressing TNC1 cells and erythrocytes were incubated for 15 min with a range of concentrations of the NSC164914 compound and the resulting dose–response curves were fit using a sigmoidal function to determine the apparent EC50. Figure 6C summarizes dose–response data obtained after exposure to this compound. Under our experimental conditions, half-maximal inhibition of the shrinkage rate was 30 μM in AQP4-vesicles and 40 μM in erythrocytes, indicating a relatively low binding affinity of the selected compounds. Among drugs identified from the first screening, three others were also analyzed by stopped-flow light scattering. Comparable concentration-inhibition data were found for the selected drugs NSC670229, NSC168597, and NSC301460 probed on red blood cells (Figure 7). Figure 8 shows the chemical structures of the selected hits confirmed by the secondary screening; all were found within the NCI mechanistic set.
Figure 6.
Effect of NSC164914 compound on plasma membrane osmotic water flow analyzed by stopped-flow light scattering. (A) Comparison of water permeability measurements for membrane vesicles obtained from TNC1 wt cells and AQP4-TNC1 cells under control conditions and in the presence of 40 μM of the indicated inhibitory compound. The histogram to the right summarizes the average rate constant values (τ, s) from three sets of experiments, each done on membrane vesicles from different preparations. *P < 0.01. (B) Measurement of water transport in red blood cells in the absence or presence of the selected compound. The average rate constant values (τ, s) from three sets of experiments for each condition, each done on blood from different rats, are plotted on the right. *P < 0.001 compared to control. (C) Dose–response curves for inhibition of water transport in erythrocytes and in plasma membrane vesicles from AQP4-expressing TNC1 cells. The compounds were added with increasing concentrations for 15 min before stopped-flow measurements. The rate constant for shrinkage is normalized relative to the rate constant in the absence of inhibitor. Data were plotted on a logarithmic scale and fitted using Prism (GraphPad) software to the sigmoidal dose–response (variable slope) equation. Every point is an average of three different preparations. NSC164914 inhibits with an EC50 of 40 μM in erythrocytes and an EC50 of 30 μM in AQP4-expressing plasma membrane vesicles.
Figure 7.
Dose–response curves for inhibition of water flow in erythrocytes by selected drugs. Erythrocytes were incubated for 15 min with compounds at the indicated concentrations prior to stopped-flow analysis. Each point is an average of three different preparations. The deduced EC50 values were 27 μM for NSC 670229, 49 μM for NSC168597, and 28 μM for NSC 301460.
Figure 8.
Chemical structures of selected compounds from the mechanistic set found effective in altering water permeability: NSC301460 trichopolin; NSC670229 2-[4-tert-butyl-1-[(4-methylphenyl)methyl] cyclohexyl]oxy-N,Ndimethylethanamine; NSC168597 tributyl-chloroplumbane; NSC164914 tributyl-(2,4,5-trichlorophenoxy)stannane.
DISCUSSION
The primary purpose of this study was to implement a 96-well plate assay in order to test chemical libraries for lead compounds that affect aquaporin (AQP) water channels. Since their identification, a number of studies have supported the involvement of the AQP family in a wide range of altered water balance disorders which can severely affect the quality of life. Most of the knowledge concerning the physiological role of AQPs has come from phenotype analysis of transgenic mouse models with AQP knockout or mutation. These studies have validated the key role of AQPs in kidney, brain, eye, skin, fat, and exocrine glands and their implication in major organ functions and diseases that are characterized by excessive water transport such as urinary concentration, brain edema, epilepsy, glaucoma, tumor growth, and obesity. The development of chemical AQP-selective modulators is, thus, predicted to provide a novel therapeutic strategy that will significantly impact the clinic.
We report here a functional medium-throughput screening assay based on the original cell microscopy calcein quenching method reported by Hamman et al.28 In this method, osmotically induced cell volume changes were deduced from changes in total fluorescence of calcein-loaded cells.28,29 In our method, real time cellular fluorescence kinetics of 96 samples were monitored by a conventional fluorescence plate reader equipped with integrated liquid handling.
Technological advances in the past few years have predicted the fluorescence-based methods as among the most important detection strategies used for high throughput screening (HTS) because of their very high sensitivity, allowing simplification, miniaturization, and increased speed of assays. In addition, screening assays that are based on optical readout including fluorescence are commonly more robust, suitable for automation, and less expensive than those utilizing radioisotopes or electrophysiology. A growing number of reports document automatic platereaders as a promising cell-based screening technology for the use of biological reagents in volumes consistent with miniaturized assay formats.
The assay protocol developed here offers a simple and reliable platform for automated compound screening without microscopy analysis and suitable for adaptation to a 384-well format. Cell volume changes due to functional AQP channels were analyzed in cells grown on 96-well microplates without any intervention between cell plating and assay, except for calcein loading and addition of test compounds. Assay development also required selection of cell lines suitable for measurement of AQP activity and screening application. The cell lines were screened for robust growth, good adherence on plastic multiwell plates and low AQP-independent water permeability. The functional assay for compound screening was initially performed on fibroblast cells and mouse astrocyte primary cultures that strongly express AQP1 and AQP4 proteins, respectively, as indicated by immunochemical studies. Experimental conditions were optimized for robustness and sensitivity, two parameters that are extremely important for drug discovery.
We first characterized the calcein quenching method in these cells to validate its applicability to water permeability measurements in response to an imposed osmotic gradient and, thus, to study the AQP-mediated water transport. Water flux kinetics recorded upon changing bath osmolality revealed fast osmotic responses of both fibroblasts and astrocytes together with a strong regulation of their cell volume, a critical mechanism that allows them to recover their original volume in the continued presence of the osmotic stress depending on the activation of cation and anion permeabilities. A correlation between AQPs and ionic channel activity has been previously reported suggesting that AQP expression might be involved in ionic transport and volume regulation processes.41–43 Further analysis showed the independence of the cell shrinkage time constant on the extracellular osmolarity as well as the correlation of the signal calcein magnitude with the size of the applied osmotic gradient in both cell types. The inhibitory effect produced by mercuric chloride on osmotic water permeability of AQP1-expressing fibroblasts was also confirmed.
Because the lipid bilayer provides an AQP4-independent water transport pathway in astrocytes, we selected the TNC1 cell line for its low basal water permeability to generate a stably transfected cell line expressing AQP4. We verified the suitability of the stable cell line for AQP4 functional assays using the automatic fluorescence plate reader and compared its water permeability with primary astrocytes. An interesting finding was that transfected cells regulated their volume toward normal when exposed to an osmotically altered environment, exhibiting strong RVD and RVI properties. This is similar to what we found in astrocytes and fibroblasts and suggests that ion channels involved in volume regulation are activated when a rapid AQP-mediated water flux occurs. All these data confirm that the calcein-based method can resolve differences in the kinetics of cell volume changes in AQP-expressing and control cells and, thus, allow computation of the contribution of AQPs to cellular water transport.
In order to test whether this multiwell plate screening strategy could be used to identify putative AQP modulators, we screened diverse collections of druglike small molecules available through the National Cancer Institute and a small library of FDA approved drugs compiled by our colleague Dr. David Lawrence (see Acknowledgment). Initial screening was performed using individual compounds at 20 μM and allowed us to select 10 compounds affecting water transport activity in both astrocytes and AQP4-TNC1 cells and 42 compounds that affect cell volume regulation in astrocytes. Interestingly, these compounds mainly belong to the same chemical set which includes compounds with a wide range of growth inhibition patterns determined in a panel of human cancer cell lines. Among the FDA-approved drugs, we also identified a number of drugs, some of which are illustrated in Figure 4 (astemizole, fluphenazine, and miconazole), that affected the regulation of cell volume in cultured astrocytes. It is well established that astrocytes in culture, like most cell types, can regulate their volume through the activation of volume-sensitive ion channels when subjected to an osmotic gradient. The intracellular osmolytes involved in regulatory volume mechanisms are inorganic ions, including K+ and Cl-, and organic molecules, such as amino acids.44,45 In hypotonic conditions, the decreases in external osmolarity lead to activation of the transmembrane release of these osmolytes, thus adjusting the intracellular concentration of osmotically active solutes.
Astemizole, a histamine H1-receptor antagonist, is a relatively nonspecific ion channel blocker that can also inhibit specific voltage gated potassium channels.46,47 It was recently implicated in tumor progression by inhibiting hEag1 channels and is involved in adverse cardiac effects including the lengthening of repolarization because of its blocking effect on HERG channels. The calmodulin antagonist fluphenazine, a typical antipsychotic drug, has recently been shown to be involved in the volume-activated channel regulatory machinery.45 Experiments performed in a lymphoma cell line indicated miconazole, a broad spectrum antimycotic, was able to modify initial volume expansion and rate of regulatory volume decrease in a dose-dependent and time-dependent manner.48 On the basis of such experimental evidence, it is plausible that these selected molecules may play an important role in cell volume regulation in response to osmotic challenge.
Few molecules directly inhibited AQP-mediated water transport. After confirming the selected hits by the fluorescence assay and verifying their ineffectiveness in parental TNC1 cells, we performed a secondary analysis by stopped-flow light scattering, an alternative technique used to better analyze the selected compounds for further development. The analysis of the chemical compounds was performed in plasma membrane vesicles obtained from AQP4 transfected cells and in erythrocytes, a simple and a widely used cell model to study AQP1 water channel activity by stopped-flow light scattering.19,49 Four molecules were selected that, at low concentration, produced substantial inhibition of water permeability in erythrocytes and AQP4-expressing vesicles. It is important to stress that, in our experimental conditions, we did not observe a significant erythrocyte hemolysis induced by these compounds that could interfere with water transport measurements.50 An interesting finding was that two of them, NSC164914 (tributyl-(2,4,5-trichlorophenoxy)stannane) and NSC168597 (tributyl-chloroplumbane), contain metals, tin and lead, respectively (Figure 8), and have inhibitory effects on both AQP4 and AQP1. Interestingly, other heavy metal ions as silver, gold,11 copper,51 and mercury19 have been reported as water channel inhibitors although these metals are extremely toxic and require high concentrations to produce significant effects.
Other compounds, with either much lower or no efficacy, may have had apparent AQP inhibitory activity in the primary screening because of their toxicity or putative effect on calcein fluorescence. Dose–response experiments carried out in plasma membrane vesicles from AQP4-expressing cells and erythrocytes to quantify the inhibitory potency of the selected hits revealed EC50 values between 30 and 40 μM, suggesting a relatively low binding affinity of the selected compounds. In order to improve potency, specificity, and pharmacological properties of selected lead compounds, we plan to define their structure–activity relationship (SAR) by evaluation of similar existing compounds and/or by generating optimized structurally related analogs. As AQP1 is close to AQP4 in terms of sequence homology,52 we can hypothesize a similar putative mechanism by which the identified molecules interact with these water channels giving rise to the albeit weak inhibitory effect found on their functional activity. The combination of molecular docking studies with subsequent molecular dynamics simulation could be an effective tool to predict and to characterize binding sites for ligand molecules. It can be speculated that the observed water transport inhibition is likely mediated through an interaction between selected hits and amino acids located at the extracellular face of AQPs, as A-, C-, and E-loops. Moreover, the molecular characterization of the putative binding sites, corroborated by mutational studies, will open the way for a rational structure-based approach to select more efficient blockers of aquaporins.
In summary, our present data establish a convenient screening procedure as a promising approach to analyze AQP-mediated water transport and investigate the effect of compounds on AQP function in order to discover potential chemical modulators of water channel activity. Suitable cell lines were generated and experimental conditions were optimized to improve functional assay sensitivity and allow the development of a novel therapeutic strategy in restoration of normal water balance in human diseases.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by Grants: MIUR-PRIN (to A.F.) 2006, Internationalization 2004–2006 (to M.S.), NS041282 (to D.C.S.), Progetto Strategico APQ Ricerca PS124 (Neurobiotech) and HD 32573 (to D.C.S.), and F.M. Kirby Foundation for the purchase of FlexStation II. We gratefully acknowledge the helpful advice and generous provision of the 418 FDA-compound library by Dr. David S. Lawrence, formerly of the Department of Biochemistry at Einstein and currently the Fred Eshelman Distinguished Professor of the Division of Medicinal Chemistry and Natural Products, School of Pharmacy, University of North Carolina.
Footnotes
SUPPORTING INFORMATION AVAILABLE
Additional information as noted in text. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. J. Physiol. 2002;542:3–16. doi: 10.1113/jphysiol.2002.020818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verkman AS. J. Membr. Biol. 2000;173:73–87. doi: 10.1007/s002320001009. [DOI] [PubMed] [Google Scholar]
- 3.Verkman AS. Ann. Med. 2002;34:192–200. [PubMed] [Google Scholar]
- 4.Manley GT, Binder DK, Papadopoulos MC, Verkman AS. Neuroscience. 2004;129:983–991. doi: 10.1016/j.neuroscience.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 5.Verkman AS. Expert Rev. Mol. Med. 2008;10:e13. doi: 10.1017/S1462399408000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadopoulos MC, Verkman AS. Prog. Brain Res. 2008;170:589–601. doi: 10.1016/S0079-6123(08)00446-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gade W, Robinson B. Clin. Lab. Sci. 2006;19:80–89. [PubMed] [Google Scholar]
- 8.Ceperuelo-Mallafré V, Miranda M, Chacón MR, Vilarrasa N, Megia A, Gutiérrez C, Fernández-Real JM, Gómez JM, Caubet E, Frühbeck G, Vendrell J. J. Clin. Endocrinol. Metab. 2007;92:3640–3645. doi: 10.1210/jc.2007-0531. [DOI] [PubMed] [Google Scholar]
- 9.Verkman AS. Rev. Physiol. Biochem. Pharmacol. 2005;155:31–55. doi: 10.1007/3-540-28217-3_2. [DOI] [PubMed] [Google Scholar]
- 10.Frigeri A, Nicchia GP, Svelto M. Curr. Pharm. Des. 2007;13:2421–2427. doi: 10.2174/138161207781368738. [DOI] [PubMed] [Google Scholar]
- 11.Niemietz CM, Tyerman SD. FEBS Lett. 2002;53:443–447. doi: 10.1016/s0014-5793(02)03581-0. [DOI] [PubMed] [Google Scholar]
- 12.Brooks HL, Regan JW, Yool AJ. Mol. Pharmacol. 2000;57:1021–1026. [PubMed] [Google Scholar]
- 13.Detmers FJ, de Groot BL, Müller EM, Hinton A, Konings IB, Sze M, Flitsch SL, Grubmüller H, Deen PM. J. Biol. Chem. 2006;281:14207–14214. doi: 10.1074/jbc.M513072200. [DOI] [PubMed] [Google Scholar]
- 14.Yool AJ, Brokl OH, Pannabecker TL, Dantzler WH, Stamer WD. BMC Physiol. 2002;2:4. doi: 10.1186/1472-6793-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma B, Xiang Y, Mu SM, Li T, Yu HM, Li X. J. Acta Pharmacol. Sin. 2004;25:90–97. [PubMed] [Google Scholar]
- 16.Gao J, Wang X, Chang Y, Zhang J, Song Q, Yu H, Li X. Anal. Biochem. 2006;350:165–170. doi: 10.1016/j.ab.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Huber VJ, Tsujita M, Yamazaki M, Sakimura K, Nakada T. Bioorg. Med. Chem. Lett. 2007;17:1270–1273. doi: 10.1016/j.bmcl.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Huber VJ, Tsujita M, Kwee IL, Nakada T. Bioorg. Med. Chem. 2009;17:418–24. doi: 10.1016/j.bmc.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 19.Yang B, Kim JK, Verkman AS. FEBS Lett. 2006;580:6679–6684. doi: 10.1016/j.febslet.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Søgaard R, Zeuthen T. Pflugers Arch. 2008;456:285–292. doi: 10.1007/s00424-007-0392-2. [DOI] [PubMed] [Google Scholar]
- 21.Yang B, Zhang H, Verkman AS. Bioorg. Med. Chem. 2008;16:7489–7493. doi: 10.1016/j.bmc.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hoek AN, de Jong MD, van Os CH. Biochim. Biophys. Acta. 1990;1030:203–210. doi: 10.1016/0005-2736(90)90296-z. [DOI] [PubMed] [Google Scholar]
- 23.Nicchia GP, Frigeri A, Liuzzi GM, Santacroce MP, Nico B, Procino G, Quondamatteo F, Herken R, Roncali L, Svelto M. GLIA. 2000;31:29–38. doi: 10.1002/(sici)1098-1136(200007)31:1<29::aid-glia30>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24.Radany EH, Brenner M, Besnard F, Bigornia V, Bishop JM, Deschepper CF. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6467–6471. doi: 10.1073/pnas.89.14.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunnarson E, Axehult G, Baturina G, Zelenin S, Zelenina M, Aperia A. Neuroscience. 2005;136:105–114. doi: 10.1016/j.neuroscience.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 26.Nicchia GP, Frigeri A, Liuzzi GM, Svelto M. FASEB J. 2003;17:1508–1510. doi: 10.1096/fj.02-1183fje. [DOI] [PubMed] [Google Scholar]
- 27.Nicchia GP, Srinivas M, Li W, Brosnan CF, Frigeri A, Spray DC. FASEB J. 2005;19:1674–1676. doi: 10.1096/fj.04-3281fje. [DOI] [PubMed] [Google Scholar]
- 28.Hamann S, Kiilgaard JF, Litman T, Alvarez-Leefmans FG, Winther BR, Zeuthen T. J. Fluoresc. 2002;12:139–145. [Google Scholar]
- 29.Oldenburg KR, Karivk I, Zhang JH, Chung TDY, Lin S. Handbook of Drug Screening. Marcel Dekkar, Inc.; New York: 2001. [Google Scholar]
- 30.Zhang JH, Chung TD, Oldenburg KR. J. Biomol. Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 31.Nicchia GP, Rossi A, Mola MG, Procino G, Frigeri A, Svelto M. GLIA. 2008;56:1755–1766. doi: 10.1002/glia.20724. [DOI] [PubMed] [Google Scholar]
- 32.van Hoek AN, Verkman AS. J. Biol. Chem. 1992;267:18267–18269. [PubMed] [Google Scholar]
- 33.Frigeri A, Nicchia GP, Balena R, Nico B, Svelto M. FASEB J. 2004;18:905–907. doi: 10.1096/fj.03-0987fje. [DOI] [PubMed] [Google Scholar]
- 34.Lu M, Lee MD, Smith BL, Jung JS, Agre P, Verdijk MA, Merkx G, Rijss JP, Deen PM. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10908–10912. doi: 10.1073/pnas.93.20.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solenov E, Watanabe H, Manley GT, Verkman AS. Am. J. Physiol. Cell Physiol. 2004;286:C426–C432. doi: 10.1152/ajpcell.00298.2003. [DOI] [PubMed] [Google Scholar]
- 36.Rothstein A, Mack E. Am. J. Physiol. 1991;260:C113–C121. doi: 10.1152/ajpcell.1991.260.1.C113. [DOI] [PubMed] [Google Scholar]
- 37.Heo J, Meng F, Sachs F, Hua SZ. Cell. Biochem. Biophys. 2008;51:21–32. doi: 10.1007/s12013-008-9010-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furman CS, Gorelick-Feldman DA, Davidson KG, Yasumura T, Neely JD, Agre P, Rash JE. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13609–13614. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicchia GP, Mastrototaro M, Rossi A, Pisani F, Tortorella C, Ruggieri M, Lia A, Trojano M, Frigeri A, Svelto M. GLIA. 2009 doi: 10.1002/glia.20855. in press. [DOI] [PubMed] [Google Scholar]
- 40.Silberstein C, Bouley R, Huang Y, Fang P, Pastor-Soler N, Brown D, Van Hoek AN. Am. J. Physiol. Renal Physiol. 2004;287:F501–F511. doi: 10.1152/ajprenal.00439.2003. [DOI] [PubMed] [Google Scholar]
- 41.Nagelhus EA, Mathiisen TM, Ottersen OP. Neuroscience. 2004;129:905–913. doi: 10.1016/j.neuroscience.2004.08.053. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Bandyopadhyay BC, Nakamoto T, Singh B, Liedtke W, Melvin JE, Ambudkar I. J. Biol. Chem. 2006;281:15485–15495. doi: 10.1074/jbc.M600549200. [DOI] [PubMed] [Google Scholar]
- 43.Galizia L, Flamenco MP, Rivarola V, Capurro C, Ford P. Am. J. Physiol. Renal Physiol. 2008;294:F582–F590. doi: 10.1152/ajprenal.00427.2007. [DOI] [PubMed] [Google Scholar]
- 44.Pasantes-Morales H, Murray RA, Lilja L, Morán J. Am. J. Physiol. 1994;266:C165–C171. doi: 10.1152/ajpcell.1994.266.1.C165. [DOI] [PubMed] [Google Scholar]
- 45.Mongin AA, Cai Z, Kimelberg HK. Am. J. Physiol. 1999;277:C823–C832. doi: 10.1152/ajpcell.1999.277.4.C823. [DOI] [PubMed] [Google Scholar]
- 46.Roy J, Vantol B, Cowley EA, Blay J, Linsdell P. Oncol. Rep. 2008;19:1511–1516. [PubMed] [Google Scholar]
- 47.Gómez-Varela D, Contreras-Jurado C, Furini S, García-Ferreiro R, Stühmer W, Pardo LA. FEBS Lett. 2006;580:5059–5066. doi: 10.1016/j.febslet.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 48.Watson PA, Giger KE, Frankenfield CM. Mol. Cell. Biochem. 1991;104:51–56. doi: 10.1007/BF00229803. [DOI] [PubMed] [Google Scholar]
- 49.Yang B, Verkman AS. J. Biol. Chem. 2002;277:36782–36786. doi: 10.1074/jbc.M206948200. [DOI] [PubMed] [Google Scholar]
- 50.Kleszcynjska H, Hładyszowski J, Pruchnik H, Przestalski S. Z. Naturforsch., C: J. Biosci. 1997;52:65–69. [PubMed] [Google Scholar]
- 51.Parisi M, Piccinni ZF. J. Endocrinol. 1972;55:1–9. doi: 10.1677/joe.0.0550001. [DOI] [PubMed] [Google Scholar]
- 52.Zardoya R, Villalba S. J. Mol. Evol. 2001;52:391. doi: 10.1007/s002390010169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.