Abstract
As clinical trials with stem cells for cardiac regenerative therapy move forward, advances in imaging equipment and technique offer powerful methods to evaluate therapeutic efficacy. Methodologies to label stem cells for tracking continue to expand. Non-invasive imaging offers the potential to better understand the interaction of exogenous stem cells with the host to answer questions such as the best cell type(s), timing of delivery, dose, and delivery route. If successful, these techniques may enable individually tailored dosing of stem cell therapeutics. However, techniques that are suitable for animal models of cardiac disease may have hurdles to clinical translation beyond simple biocompatibility issues. Challenges include the high cost of advanced imaging techniques, applicability in acute ischemic disease, and regulatory approval. In this review, we will cover some new imaging techniques and labeling strategies and assess the obstacles to clinical adoption.
Keywords: Magnetic Resonance Imaging, Radionuclide Imaging, CT Imaging, Stem Cells, Reporter Gene Imaging
Introduction
Nearly all cardiovascular stem cell trials of ischemic heart failure or acute myocardial infarction have used at least one imaging modality (and often several imaging modalities) to assess patient response to treatment [6, 7, 16, 18, 20, 28, 34, 43]. Two-dimensional echocardiography provides an inexpensive, clinically accepted method for evaluating cardiac function without ionizing radiation. On the other hand, X-ray angiography remains the gold standard for interrogation of vessel patency. Thus, these two techniques dominate the imaging modalities that have been used in clinical trials. However, both techniques suffer from limited imaging windows and cannot provide three-dimensional imaging, which can be important in assessing left ventricular remodeling after infarction.
Three-dimensional echocardiography can provide more complete anatomy compared to traditional transthoracic echocardiography, but is more invasive and requires patient sedation. Using a relatively new echocardiographic technique, Janssens and colleagues demonstrated that strain-rate imaging can be used to demonstrate improvements in regional cardiac function in patients with ST-elevation myocardial infarction receiving bone marrow progenitor cells that cannot be recognized using global function parameters, such as left ventricular ejection fraction [18]. Clinical trials, such as the PROSPECT trial [11] (which was unable to predict responders to ventricular resynchronization based on echocardiograpic measures), raise concern as to whether the results from the trial by Janssens and coworkers studying a limited number of patients will be replicated in a larger scale clinical trial or whether these techniques require further refinement [33].
Meta-analyses of the clinical trials, to date, indicate that stem cell therapy in cardiac patients is safe and may provide slight improvements in cardiac function [1, 15, 27, 36, 46]. Nonetheless, should larger scale clinical trials fail to confirm the results of these initial trials, it would be useful to be able to use imaging to possibly distinguish non-responders from responders following therapy. In addition, labeled stem cells that could be tracked noninvasively could provide a means of identifying patients where stem cell delivery, engraftment, or survival was suboptimal to provide better treatment strategies rather than abandoning the therapy as not efficacious.
Although trials using various labeling methods are limited, these initial results are promising. In the first clinical trial using a combination of superparamagnetic iron oxides (SPIOs) and radiotracer cell labels, de Vries et al. demonstrated that ultrasound-guided lymph node injections failed to reach the targeted nodes in 50% of the patients [14]. Only magnetic resonance imaging (MRI), a tomographic or 3D technique, and not nuclear scintigraphy was capable of demonstrating these errors in cell targeting [14]. Tracking of SPIO-labeled stem cells in stroke [47] gave further excitement that rapid translation of cardiac stem cell therapy could be realized. MRI, however, encountered several major obstacles. First, MRI is costly, time consuming, and often impractical in cardiac patients, who often have devices, such as pacemakers, that are not MRI compatible. Unlike direct labeling with radioactive substances where the half-life of the tracer severely limits the ability to perform serial tracking, concerns have been raised that SPIOs used to label stem cells may persist long after the exogeneously administered stem cells have died [2, 26, 39]. Thus, following SPIO labeling of stem cells, it may not be possible to determine whether the MR signal originates from viable cells or from the persistence of the cell label after cell death. Reporter genes offer a potential alternative means to track cells with non-invasive imaging without some of the pitfalls of false positive and negative cell tracking that may occur with direct cell labeling. Clinical and public acceptance of genetically manipulated cells has been limited since the unfortunate death of a patient enrolled in a gene therapy trial in September 1999 [42]. Nonetheless, the Food and Drug Administration approved the first clinical trial using embryonic stem cells for spinal cord injury [12], and clinical trials of genetically altered T cells in oncology patients are ongoing [45]. These developments suggest that support for therapies that incorporate genetic alteration is growing, and this momentum will facilitate clinical translation of new techniques in cell tracking and non-invasive imaging, which we will highlight in this review.
New Advances in Reporter Gene Imaging
In the past year, there have been a variety of preclinical studies exploring several aspects of cardiac stem cell therapy. Historically, exogenously expressed green fluorescent protein (GFP) has been ubiquitously used as a reporter gene in histological specimens. However, the attenuation of fluorescence across tissue layers limits GFP utility as an in vivo optical imaging reporter gene. For in vivo reporter gene imaging, bioluminescence imaging (BLI) is commonly used to track cells transfected to express firefly luciferase. The administration of the reporter probe, D-luciferin, in the presence of ATP is catalyzed by luciferase to oxyluciferin with the release of light that can be recorded by a charge-coupled device camera.
Using BLI, preferential homing of bone marrow mononuclear (BMMN) cells to mouse hearts subjected to ischemia/reperfusion after intravenous injection could be demonstrated compared to sham animals over a 4-week period [35]. The survival of different cell types has also been serially imaged using BLI. In immunocompetant mice with a non-reperfused infarction, BMMN cells showed a distinct survival advantage over mesenchymal stem cells, fibroblasts, or skeletal myoblasts after direct intramyocardial injection [41]. The enhanced survival of BMMN cells was also correlated with improved preservation of myocardial shortening and less ventricular remodeling by echocardiography [41]. In a similar study, cardiac stem cells (CSC) injected intramyocardially in a non-reperfused murine myocardial infarction showed poor engraftment and survival at 8 weeks [25]. In contrast, mouse studies where human cardiosphere-derived cells (CDC) were injected in the infarct border zone showed more viable myocardium in the infarct zone suggesting migration of the hCDCs [37]. However, no in vivo method was used to track the CDCs. In a similar study, intracoronary administration of hCDCs to swine following acute myocardial infarction showed attenuation of infarct size and concurrent formation of new cardiac tissue at 8 weeks [21]. However, no in vivo CDC tracking was performed in this study either. Thus, reporter gene imaging may be useful in teasing out differences in stem cell preparations and administration that has heretofore led to contradictory results.
While bioluminescence imaging has dominated the reporter gene imaging in recent years, there are several other reporter genes that have been developed for use with radionuclide imaging [10, 29, 44]. Recently, Abraham and coworkers have demonstrated that, up to 6 days post-injection, they were able to track CSCs expressing the sodium iodide symporter (NIS), a naturally occurring membrane glycoprotein that is expressed in a limited number of organs, with single-photon emission tomography (SPECT) or positron emission tomography (PET) [38]. Since a major limitation with any reporter gene method is the immune response elicited toward the production of a foreign protein/enzyme, immunogenicity from endogenous NIS gene product should be quite limited, and this makes it an attractive reporter gene system for further clinical translation in the realm of cardiac stem cell therapy. However, the effects of additional sodium channels on the function of cells in the heart is not fully characterized. In other methods, notably SPECT and PET, variants of the thymidine kinase reporter gene have dominated reporter gene imaging, to date. Gyöngysi and colleagues have initiated the first successful translation of thymidine kinase reporter gene imaging for cardiac stem cell tracking of biodistribution in a relevant large animal model of myocardial infarction. These authors used a minimally invasive stem cell delivery method of electromechanical mapping for transendocardial delivery, combined with clinical PET imaging (Fig. 1) [17].
Fig. 1.
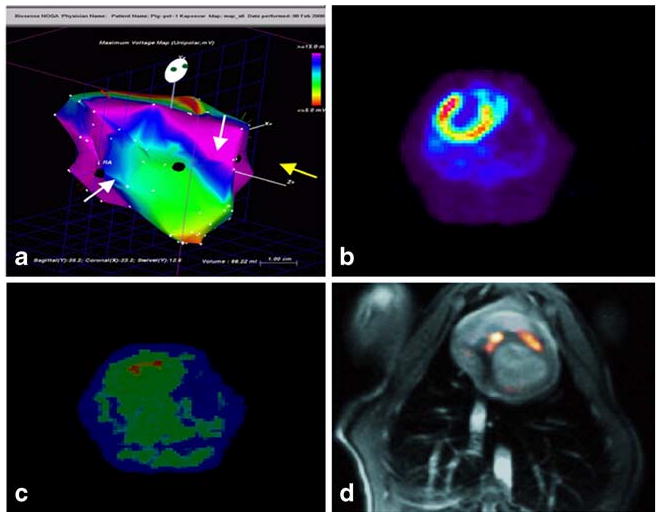
Electromechnical-mapping-guided mesenchymal stem cell delivery in a swine myocardial infarction (MI) model. a Endocardial mapping of a pig heart 16 days after MI. MSCs transfected with a truncated thymidine kinase reporter gene were intramyocardially injected into the border zone of the infarction (white arrows), and unlabeled MSCs were delivered into noninfarcted posterior wall (yellow arrow). b 13N-ammonia positron emission tomography with transmission scan of the pig heart showing perfusion defect in the anterior wall and apex 16 days after MI. c The location of two injection sites of reporter gene transfected MSCs are demonstrated by18F-FHBG PET image of the pig heart 8 h after injection. Unlabeled MSCs could not be detected. d Registration of 18F-FHBG PET (hot scale) with MRI (gray scale) demonstrating tracer uptake only at MSCs injection sites. Reprinted from Gyöngysi et al. [17] with permission
New Advances in Iron Oxide Stem Cell Labeling
Iron oxide stem cell labeling for MRI tracking remains one of the most commonly used methods in preclinical studies for cell tracking. Controversy continues about whether the hypointensities from iron oxides seen by MRI represent viable exogenously labeled stem cells or residual iron freed from dying stem cells [2, 8, 9, 19, 26, 32, 39]. The biggest obstacle to clinical translation of SPIO-based therapies is that many of the formulations of iron oxides and ferumoxides that were once used in limited clinical practice are no longer commercially available. Thus, enthusiasm for clinical adoption of these techniques has markedly diminished.
New Advances in Microencapsulation for Cardiovascular Cellular Imaging
Microencapsulation of cells was originally developed as a means to avoid immunosuppressive therapies for allogeneic or xenogeneic transplantation therapy—particularly for islet cell transplantation in type I diabetic patients [13, 31]. Our group realized the potential to incorporate contrast agents within the microcapsule to enable cell tracking [3, 4]. Because the contrast agent is contained in the microcapsule rather than direct labeling of the stem cell, radiopaque contrast, which would typically be cytotoxic, can be utilized to enable tracking by conventional X-ray angiography and cardiac computed tomography (CCT). Improvements in multidetector CT have lead to increased utilization of CCT for diagnostic imaging of cardiovascular patients. Thus, microencapsulation of stem cells with radiopaque contrast agents could enable serial non-invasive imaging of stem cell delivery using conventional clinical X-ray equipment.
In addition to preventing immunorejection by providing a semiporous membrane that restricts the ability of immuno-globins and immune-mediated cells to destroy transplanted cells, microencapsulation may also offer a better niche for transplanted cells by providing a surface for cell adhesion and improved cell-to-cell contact. Initial studies in our group explored the modification of the classical alginate-poly-l-lysine-alginate (APA) encapsulation method with the addition of 12% barium sulfate to enable tracking of mesenchymal stem cells (MSCs) using clinical X-ray fluoroscopic systems in a rabbit model of peripheral arterial disease (Fig. 2) [22–24, 30]. Subsequently, our group has explored the addition of perfluoroctylbromide (PFOB) to the APA microcapsule to enable multi-modality imaging. The fluorine moiety of PFOB may be used for 19F MRI, whereas the perfluorocarbon can be used as an acoustic shadow for ultrasonic imaging. Finally, the bromide provides radiopacity for c-arm CT. In addition, perfluorocarbons, which have been used as blood substitutes, may enhance oxygen tension in the ischemic environment.
Fig. 2.
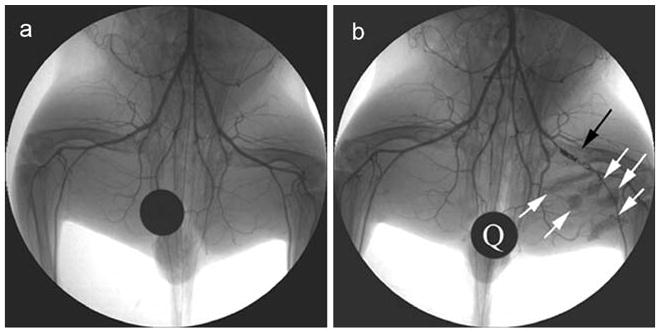
X-ray angiogram of the rabbit peripheral hindlimb before intervention (a) and after femoral artery occlusion using a platinum coil (black arrow; b). X-ray visible, mesenchymal stem cells containing microcapsules appear as radiopacities in the medial thigh of the rabbit after intramuscular delivery (b). Q quarter for reference of size and opacity. Reprinted from Nahrendorf et al. [30] with permission
Preliminary studies have demonstrated that the addition of PFOB does not alter the diffusive properties of the microcapsule, such that diffusion of nutrients including oxygen and waste products is not altered. In addition, in vitro studies indicate that the addition of PFOB does not alter MSC viability. Furthermore, if PFOB-APA encapsulation is performed using MSCs transfected with reporter genes, MSC viability within the microcapsule can be determine using non-invasive imaging (Fig. 3) [40]. Thus, microencapsulation in combination with radiopaque contrast agents may provide a method to track stem cells using a well-accepted X-ray fluoroscopic imaging platform commonly used in cardiovascular application. However, further optimization of the microencapsulation techniques will be necessary since the large size of the microcapsules may lead to embolic events after intracoronary administration or conduction abnormalities after intramyocardial administration.
Fig. 3.
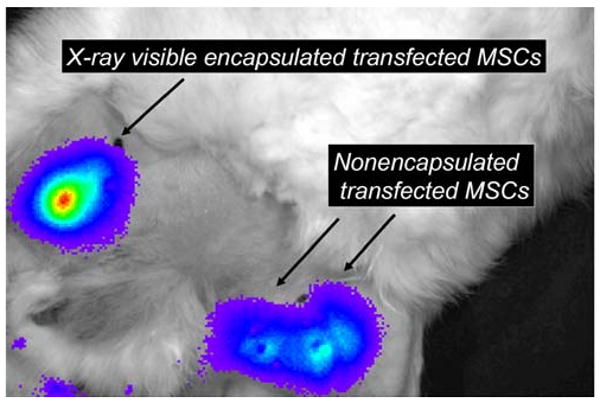
Bioluminescence images of the medial thigh of a rabbit model of peripheral arterial disease provides the ability to assess cell viability in vivo of intramuscularly injected X-ray-visible encapsulated mesenchymal stem cells that were transfected with a reporter gene as well as nonencapsulated reporter gene transfected MSCs. Reprinted from Tsui and Kraitchman [40] with permission
Conclusions
Techniques for stem cell labeling are now well established for preclinical studies. The most promising methods, such as iron oxide stem cell labeling, are currently hindered by issues related to concerns about the stem cell label becoming dissociated from the exogenously labeled stem cell—an issue which plagues most direct labeling techniques. However, these techniques still offer a method for determining the immediate success of stem cell delivery even if serial inspection of stem cell persistence may be impaired [5]. Reporter gene imaging offers the only non-invasive means to determine stem cell viability. Whereas reporter gene expression is often short-lived, this may prove to alleviate concerns about long-term expression of a foreign protein or enzyme. Microencapsulation techniques offer a method for X-ray tracking of stem cells. However, due to the large size of the therapeutic product, these techniques are currently limited to cardiovascular studies outside the heart. While the benefit of stem cell labeling appears obvious, the hurdle will be to convince regulatory agencies as well as clinical staff that the ability to determine the success of stem cell delivery and tracking of engraftment will outweigh the effort and time associated with stem cell labeling. Thus, these techniques may not only provide the means to study the conflicting responses of individual patients, but also to tailor therapies for each patient to enable an optimal response to treatment.
Acknowledgments
This work was supported by grants from Siemens Medical Systems, the National Institutes of Health (R21HL89029), and the Maryland Stem Cell Research Fund (2008-MSCRFII-0399). Research materials were provided from Boston Scientific Corp, Inc. The authors thank Brad Barnett, Dr. Jeff W. M. Bulte, and Dr. Aravind Arepally for assistance with the microencapsulation design and Drs. Steve Shea, Tina Ehtiati, Ron Ouwerwerk, and Robert Krieg for assistance with the medical imaging.
References
- 1.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: A systematic review and meta-analysis. Archives of Internal Medicine. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 2.Amsalem Y, Mardor Y, Feinberg MS, Landa N, Miller L, Daniels D, et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation. 2007;116(suppl I):I-38–I-45. doi: 10.1161/CIRCULATIONAHA.106.680231. [DOI] [PubMed] [Google Scholar]
- 3.Barnett BP, Arepally A, Karmarkar PV, Qian D, Gilson WD, Walczak P, et al. Magnetic resonance-guided, real-time targeted delivery and imaging of magnetocapsules immunoprotecting pancreatic islet cells. Nature Medicine. 2007;13(8):986–991. doi: 10.1038/nm1581. [DOI] [PubMed] [Google Scholar]
- 4.Barnett BP, Kraitchman DL, Lauzon C, Magee CA, Walczak P, Gilson WD, et al. Radiopaque alginate microcapsules for X-ray visualization and immunoprotection of cellular therapeutics. Molecular Pharmaceutics. 2006;3(5):531–538. doi: 10.1021/mp060056l. [DOI] [PubMed] [Google Scholar]
- 5.Bartunek J, Sherman W, Vanderheyden M, Fernandez-Aviles F, Wijns W, Terzic A. Delivery of biologics in cardiovascular regenerative medicine. Clinical Pharmacology and Therapeutics. 2009;85(5):548–552. doi: 10.1038/clpt.2008.295. [DOI] [PubMed] [Google Scholar]
- 6.Bartunek J, Vanderheyden M, Vandekerckhove B, Mansour S, De Bruyne B, De Bondt P, et al. Intracoronary injection of cd133-positive enriched bone marrow progenitor cells promotes cardiac recovery after recent myocardial infarction: Feasibility and safety. Circulation. 2005;112(9 Suppl):I178–I183. doi: 10.1161/CIRCULATIONAHA.104.522292. [DOI] [PubMed] [Google Scholar]
- 7.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, et al. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (topcare-ami): Mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108(18):2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 8.Carr CA, Stuckey DJ, Tatton L, Tyler DJ, Hale SJ, Sweeney D, et al. Bone marrow-derived stromal cells home to and remain in the infarcted rat heart, but fail to improve function: An in vivo cine-mri study. Am J Physiol Heart Circ Physiol. 2008;295:H533–H542. doi: 10.1152/ajpheart.00094.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen IY, Greve JM, Gheysens O, Willmann JK, Rodriguez-Porcel M, Chu P, et al. Comparison of optical bioluminescence reporter gene and superparamagnetic iron oxide mr contrast agent as cell markers for noninvasive imaging of cardiac cell transplantation. Mol Imaging Biol. 2009;11(3):178–187. doi: 10.1007/s11307-008-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen IY, Wu JC, Min JJ, Sundaresan G, Lewis X, Liang Q, et al. Micro-positron emission tomography imaging of cardiac gene expression in rats using bicistronic adenoviral vector-mediated gene delivery. Circulation. 2004;109(11):1415–1420. doi: 10.1161/01.CIR.0000121727.59564.5B. Epub 2004 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to crt (prospect) trial. Circulation. 2008;117(20):2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 12.Couzin J. Biotechnology. Celebration and concern over U.S. Trial of embryonic stem cells. Science. 2009;323(5914):568. doi: 10.1126/science.323.5914.568. [DOI] [PubMed] [Google Scholar]
- 13.De Vos P, De Haan BJ, Wolters GH, Strubbe JH, Van Schilfgaarde R. Improved biocompatibility but limited graft survival after purification of alginate for microencapsulation of pancreatic islets. Diabetologia. 1997;40(3):262–270. doi: 10.1007/s001250050673. [DOI] [PubMed] [Google Scholar]
- 14.De Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, Van Krieken JH, Boerman OC, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nature Biotechnology. 2005;23(11):1407–1413. doi: 10.1038/nbt1154. [DOI] [PubMed] [Google Scholar]
- 15.Fan L, Chen L, Chen X, Fu F. A meta-analysis of stem cell mobilization by granulocyte colony-stimulating factor in the treatment of acute myocardial infarction. Cardiovascular Drugs and Therapy. 2008;22(1):45–54. doi: 10.1007/s10557-007-6072-9. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Aviles F, San Roman JA, Garcia-Frade J, Fernandez ME, Penarrubia MJ, De La Fuente L, et al. Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction. Circulation Research. 2004;95(7):742–748. doi: 10.1161/01.RES.0000144798.54040.ed. [DOI] [PubMed] [Google Scholar]
- 17.Gyöngysi M, Blanco J, Marian T, Trón L, Petneházy Ö, Petrasi Z, et al. Serial non-invasive in vivo positron emission tomographic (pet) tracking of percutaneously intramyocardially injected autologous porcine mesenchymal stem cells modified for transgene reporter gene expression. Circ Cardiovasc Imaging. 2008;1:94–103. doi: 10.1161/CIRCIMAGING.108.797449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbots L, D'hooge J, Eroglu E, Thijs D, Ganame J, Claus P, et al. Improved regional function after autologous bone marrow-derived stem cell transfer in patients with acute myocardial infarction: A randomized, double-blind strain rate imaging study. European Heart Journal. 2009;30(6):662–670. doi: 10.1093/eurheartj/ehn532. [DOI] [PubMed] [Google Scholar]
- 19.Higuchi T, Anton M, Dumler K, Seidl S, Pelisek J, Saraste A, et al. Combined reporter gene pet and iron oxide MRI for monitoring survival and localization of transplanted cells in the rat heart. Journal of Nuclear Medicine. 2009;50(7):1088–1094. doi: 10.2967/jnumed.108.060665. [DOI] [PubMed] [Google Scholar]
- 20.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with st-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367(9505):113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 21.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, et al. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120(12):1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. 7 p following 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kraitchman DL, Arepally A, Barnett BP, Cosby K, Gilson WD, Hofmann LV, et al. An X-ray visible microencapsulation method to enhance delivery and engraftment of allogeneic stem cells for cardiovascular applications. Contrast Media Mol Imaging. 2007;2(6):294. [Google Scholar]
- 23.Kraitchman DL, Bulte JW. In vivo imaging of stem cells an beta cells using direct cell labeling and reporter gene methods. Arterioscler Thromb Vasc Biol. 2009;29:1025. doi: 10.1161/ATVBAHA.108.165571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraitchman DL, Kedziorek DA, Gilson WD, Cosby K, Huang G, Barnett BP, et al. Encapsulated x-ray visible stem cells for arteriogenic therapy in peripheral arterial disease. Journal of Vascular and Interventional Radiology. 2008;19(2):S67. [Google Scholar]
- 25.Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, et al. Imaging survival and function of transplanted cardiac resident stem cells. Journal of the American College of Cardiology. 2009;53(14):1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Z, Suzuki Y, Huang M, Cao F, Xie X, Connolly AJ, et al. Comparison of reporter gene and iron particle labeling for tracking fate of human embryonic stem cells and differentiated endothelial cells in living subjects. Stem Cells. 2008;26(4):864–873. doi: 10.1634/stemcells.2007-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. Journal of the American College of Cardiology. 2007;50(18):1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. New England Journal of Medicine. 2006;355(12):1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 29.Miyagawa M, Beyer M, Wagner B, Anton M, Spitzweg C, Gansbacher B, et al. Cardiac reporter gene imaging using the human sodium/iodide symporter gene. Cardiovascular Research. 2005;65(1):195–202. doi: 10.1016/j.cardiores.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Nahrendorf M, Sosnovik D, French B, Swirski FK, Bengel FM, Sadeghi M, et al. Multimodality cardiovascular molecular imaging—Part II. Circulation Cardiovascular Imaging. 2009;2:56–70. doi: 10.1161/CIRCIMAGING.108.839092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'shea GM, Goosen MF, Sun AM. Prolonged survival of transplanted islets of langerhans encapsulated in a biocompatible membrane. Biochimica et Biophysica Acta. 1984;804(1):133–136. doi: 10.1016/0167-4889(84)90107-1. [DOI] [PubMed] [Google Scholar]
- 32.Pawelczyk E, Jordan EK, Balakumaran A, Chaudhry A, Gormley N, Smith M, et al. In vivo transfer of intracellular labels from locally implanted bone marrow stromal cells to resident tissue macrophages. PLoS ONE. 2009;4(8):e6712. doi: 10.1371/journal.pone.0006712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanderson JE. Echocardiography for cardiac resynchronization therapy selection: Fatally flawed or misjudged? Journal of the American College of Cardiology. 2009;53(21):1960–1964. doi: 10.1016/j.jacc.2008.12.071. [DOI] [PubMed] [Google Scholar]
- 34.Schachinger V, Assmus B, Britten MB, Honold J, Lehmann R, Teupe C, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction: Final one-year results of the TOPCARE-AMI trial. Journal of the American College of Cardiology. 2004;44(8):1690–1699. doi: 10.1016/j.jacc.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Sheikh AY, Lin SA, Cao F, Cao Y, Van Der Bogt KE, Chu P, et al. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25(10):2677–2684. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh S, Arora R, Handa K, Khraisat A, Nagajothi N, Molnar J, et al. Stem cells improve left ventricular function in acute myocardial infarction. Clinical Cardiology. 2009;32(4):176–180. doi: 10.1002/clc.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, et al. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115(7):896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 38.Terrovitis J, Kwok KF, Lautamaki R, Engles JM, Barth AS, Kizana E, et al. Ectopic expression of the sodium-iodide symporter enables imaging of transplanted cardiac stem cells in vivo by single-photon emission computed tomography or positron emission tomography. Journal of the American College of Cardiology. 2008;52(20):1652–1660. doi: 10.1016/j.jacc.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117(12):1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]
- 40.Tsui BM, Kraitchman DL. Recent advances in small-animal cardiovascular imaging. Journal of Nuclear Medicine. 2009;50(5):667–670. doi: 10.2967/jnumed.108.058479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, et al. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118(14 Suppl):S121–S129. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenner M. Tribulations of a trial. Scientific American. 2009;301(3):14–15. doi: 10.1038/scientificamerican0909-14. [DOI] [PubMed] [Google Scholar]
- 43.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The boost randomised controlled clinical trial. Lancet. 2004;364(9429):141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 44.Wu JC, Inubushi M, Sundaresan G, Schelbert HR, Gambhir SS. Positron emission tomography imaging of cardiac reporter gene expression in living rats. Circulation. 2002;106(2):180–183. doi: 10.1161/01.cir.0000023620.59633.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yaghoubi SS, Jensen MC, Satyamurthy N, Budhiraja S, Paik D, Czernin J, et al. Noninvasive detection of therapeutic cytolytic t cells with 18f-fhbg pet in a patient with glioma. Nat Clin Pract Oncol. 2009;6(1):53–58. doi: 10.1038/ncponc1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang SN, Sun AJ, Ge JB, Yao K, Huang ZY, Wang KQ, et al. Intracoronary autologous bone marrow stem cells transfer for patients with acute myocardial infarction: A meta-analysis of randomised controlled trials. Int J Cardiol. 2008;136:178–185. doi: 10.1016/j.ijcard.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J, Zhou L, Xingwu F. Tracking neural stem cells in patients with brain trauma. New England Journal of Medicine. 2006;355(22):2376–2378. doi: 10.1056/NEJMc055304. [DOI] [PubMed] [Google Scholar]


