Abstract
Liver regeneration after 70% partial hepatectomy (PH) in rats induces >95% of hepatocytes to undergo two rounds of semisynchronous cell replication. Gene expression is controlled primarily by posttranscriptional processing, including changes in mRNA stability. However, the translational activity of a specific mRNA can also be modulated after PH, resulting in significant uncoupling of protein and transcript levels relative to quiescent liver for many genes including c-myc and p53. Although the precise mechanism by which this uncoupling occurs is unknown, the polysomal association of mRNA and microRNA (miRNA) can significantly modulate rate of decay as well as translational activity. Thus we characterized the association of c-myc and p53 mRNAs and miRNAs in free and cytoskeleton- and membrane-bound polysome populations 3, 6, and 24 h after PH. The transcripts for c-myc and p53 were differentially distributed in the three discrete polysome populations, and this was dramatically modulated during liver regeneration. Nascent polysome-associated p53 and c-myc proteins were also differentially expressed in the free and cytoskeleton- and membrane-bound polysomes and significantly uncoupled from transcript levels relative to nonresected liver. At least 85 miRNAs were associated with the three polysome populations, and their abundance and distribution changed significantly during liver regeneration. These data suggest that posttranscriptional control of c-myc and p53 protein expression is associated with the translocation of transcripts between the different polyribosomes. The alteration of expression for the same transcript in different polysome populations may, in part, be due to the action of miRNAs.
Keywords: c-myc, liver regeneration, p53
after 70% partial hepatectomy (PH), mammalian livers regenerate as hepatocytes reenter the cell cycle in a fairly synchronized manner (11, 32, 37). In rats, the basal rate of DNA synthesis by hepatocytes is unchanged in the first 12 h (prereplicative phase) after PH, followed by a wave of DNA synthesis, which peaks around 24 h and then gradually declines (16). Typically, mitosis follows within 6–8 h. DNA synthesis in Ito, bile ductular, and other nonparenchymal cells is usually delayed by ∼24 h. One to several rounds of DNA synthesis takes place, with each successive round exhibiting less synchrony. In a young adult rat as many as 95% of hepatocytes undergo at least a single cycle of replication to restore the original liver mass.
Liver regeneration offers a unique in vivo model to study the regulation of gene expression in both normal and abnormal growth states. After PH transcripts for many different genes are induced, with the total amount of cytoplasmic mRNAs significantly increased during the first 12 h. The actual rate of RNA synthesis is increased only for the first several hours in liver regeneration, and many of the induced transcripts exhibit no substantial change in transcriptional activity. In fact, numerous genes expressed in the regenerating liver are regulated at the posttranscriptional level, including those modulated during the immediate-early phase (23, 33, 39). Posttranscriptional control of mRNA abundance can occur at the level of RNA processing, nuclear export of mature mRNAs, and mRNA stability (25). In addition, the intracellular localization of mRNAs also plays a role in regulating gene expression (3, 7, 15). Association of mRNAs with ribosomes or polysomes is necessary but not sufficient for translation. To ensure a spatially restricted protein distribution, it is generally thought that mRNA translation is repressed during transport and later activated on arrival at its destination by relevant signals (35).
MicroRNAs (miRNAs) are a class of ∼22-nucleotide-long RNAs that exert their functions primarily by inhibiting the expression of protein-encoding genes. miRNAs, along with associated proteins, bind to partially complementary sequences in the 3′-untranslated regions (UTRs) of their target mRNAs. The binding results in translation inhibition and/or mRNA destabilization, although the detailed mechanisms remain to be worked out. miRNAs are known to associate with polysomes, consistent with their roles in translation regulation. miRNAs are also present at various ribosome-free, cytoplasmic structures, although the fraction of miRNAs in such structures is considered low.
During liver regeneration, the steady-state c-myc and p53 mRNA and protein levels are markedly uncoupled from 50- and 35-fold to 4- and 7-fold increases, respectively (10, 33). In this study we examined whether this uncoupling is related to the association of transcripts with specific polysome populations. We fractionated rat liver polysomes into free and cytoskeleton- and membrane-bound populations from quiescent nonresected liver and from regenerating liver 3, 6 and 24 h after 70% PH. We then identified the polysome distribution of c-myc and p53 at both the transcript and protein levels. We also determined miRNA profiles associated with the three polysome populations and their modulation in regenerating liver. Our results indicate that the levels of c-myc and p53 transcripts and protein are differentially distributed and dynamically modulated in the three polysomes during liver regeneration. Moreover, uncoupling of transcript and protein levels was observed relative to time 0 h and was transcript-, polysome-, and time dependent. Profiling identified 85 miRNAs associated with the three polysome populations in rat liver. During liver regeneration, individual miRNAs changed relative abundance in the different polysomes, although the levels did not directly mirror their abundance in total RNA. Together, our data suggest that transcripts differentially associate with different polysome populations, and translational uncoupling may, in part, be due to miRNAs.
MATERIALS AND METHODS
Partial hepatectomies and tissue harvesting.
Male Sprague-Dawley rats (Harlan Sprague-Dawley, Indianapolis, IN) weighing 160–175 g were maintained on a 12:12-h light-dark cycle and fed standard laboratory chow ad libitum. Animals were subjected to 70% PH under ether anesthesia between 8 AM and noon as originally described (18). In brief, a 2-cm midventral laporatomy was performed, the liver was expelled from the body cavity by gentle pressure, and silk suture was used to tie off the left and midventral lobes, which were then surgically removed. At various times after PH, the animals were killed by exsanguination under ether anesthesia. The liver remnant was removed, rinsed in normal saline, cut into ∼0.5-cm cubes, and used immediately or flash-frozen in liquid nitrogen and stored at −80°C until use. All animal work was conducted according to protocols submitted to and approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Polysome isolation and characterization.
Cellular polysomes were fractionated and isolated with a combination of several protocols (4, 9, 40). In short, liver tissue was flash-frozen in liquid nitrogen to arrest protein synthesis and preserve ribosome-mRNA interaction. The tissue was pulverized to a fine powder in liquid nitrogen by mortar and pestle on dry ice, and this material was resuspended in 4 vols of low-salt lysis buffer (40) (0.25 M sucrose, 25 mM KCl, 5 mM MgCl2, and 10 mM ethanolamine, pH 7.4) containing 0.05% Nonidet P-40 (NP-40), 150 μg/ml cycloheximide, 100 U/ml RNase inhibitor, and Complete EDTA-free protease inhibitor (Roche) at the concentration specified by the manufacturer. After a 10-min incubation at 4°C, the tissue suspension was centrifuged at 3,000 g and the supernatant containing free polysomes decanted. The pellet was washed twice in lysis buffer and resuspended in 4 vols of cytoskeleton-bound polysome isolation buffer with 150 μg/ml cycloheximide, 100 U/ml RNase inhibitor, and Complete EDTA-free protease inhibitor. After a 15-min incubation at 4°C, the tissue suspension was centrifuged at 3,000 g and the supernatant containing cytoskeleton-bound polysomes decanted. After the pellet was washed twice with cytoskeleton-bound polysome isolation buffer, the pellet was resuspended in membrane-bound polysome isolation buffer containing 150 μg/ml cycloheximide and Complete EDTA-free protease inhibitor. After a 10-min incubation at 4°C, the tissue suspension was centrifuged at 3,000 g and the supernatant containing the membrane-bound polysomes recovered. The three different supernatants were layered over 30% sucrose pads and centrifuged at 130,000 g for 2.5 h to remove contaminating free mRNA (2). There were no differences in final yields or purity of the three polysome populations isolated from fresh (9) or frozen tissue. To confirm the polysome-dependent association of the mRNAs and nascent proteins with the individual fractions, isolations were also performed by adding 25 mM EDTA to the supernatants followed by a 15-min incubation at 4°C to dissociate the ribosomes before layering over the 30% sucrose pads and 130,000 g centrifugation. The pellets recovered from the EDTA-treated fractions were substantially reduced and yielded 14.4 ± 0.6% (P < 0.001) of the RNA or protein from the same fraction not treated with EDTA.
Contamination of the cytoskeleton- and membrane-bound fractions with free polysomes was determined by assaying the fractions before ultracentrifugation for the cytosolic marker enzyme lactate dehydrogenase with colorimetric diagnostic kit 500 (Sigma) according to the manufacturer's protocol (40). Total protein in the fractions was determined by the Bradford method with the Bio-Rad reagent as specified by the manufacturer.
RNA isolation and Competimer RT-PCR of c-myc and p53.
After ultracentrifugation the supernatant and 30% sucrose pad were removed and the tube was carefully swabbed out with sterile cotton-tipped applicators before the polysome pellet resuspension in a 2-ml Dounce with the B pestle. The material was divided into several aliquots, and one was used immediately for total RNA isolation (38), with the remaining aliquots flash-frozen in liquid nitrogen and stored at −80°C. For the EDTA-treated samples, the substantially reduced pellets after resuspension were divided in half, with one aliquot used for RNA isolation and the other processed for Western blot analyses as outlined below.
Competimer RT-PCR (Ambion) using 18S rRNA as an internal control was performed with the enzyme, nucleotide, buffer, and reaction conditions recommended by the manufacturer. All cDNA reactions were performed with 2 μg of total RNA as template and the random primers included in the RETROscript (Ambion) cDNA synthesis. Cycle number, cDNA template input, and Competimer-to-18S primer ratios were empirically determined in both single and multiplex reactions, including the p53 and c-myc primers as outlined in the Competimer manual and using 10–250 ng of input cDNA. In the final reactions, 10, 25, and 250 ng of cDNA template, 18S-to-Competimer primer ratios of 1:3, and 30 cycles of amplification were used, under conditions of 3-min denaturation at 94°C, 30 × (94°C for 45 s, 55°C for 20 s, 72°C for 45 s), followed by 5 min at 72°C. Equal amounts of the reaction products from each set of three were analyzed on 1.5% agarose gels, stained with ethidium bromide, and visualized with UV light, and images were captured. Data analysis was derived from all the reactions after normalization with the 18S rRNA signal.
Immunoblot analysis.
Total polysomal protein was prepared after ultracentrifugation by resuspending the polysome pellet by Dounce in 10 mM Tris (pH 7.6) containing 1.5 mM potassium acetate, 2 mM dithiothreitol, 0.5% NP-40, 10 mM EDTA, and Complete protease inhibitors and placing on ice for 10 min to dissociate the polysomes. After sonication and centrifugation at 5,000 g, the protein concentration of the supernatant was determined by the Bradford method with Bio-Rad reagent (Bio-Rad Laboratories). Total protein extracts (150 μg) were separated by 7.5% SDS polyacrylamide gel electrophoresis and transferred to nylon membranes. Immunoblots were processed as previously described (38) with primary antibodies (Santa Cruz) reactive against actin, β-tubulin, c-myc, p53, and vimentin at a 1:400 dilution and Abcam primary antibodies against ribosomal protein L26, the endoplasmic reticulum marker calnexin, and tubulin at 1:1,000 dilution. Secondary goat anti-mouse (actin, β-tubulin, p53, vimentin) or goat anti-rabbit (c-myc, ribosomal protein L26, calnexin, tubulin) horseradish peroxidase-conjugated antibodies (Bio-Rad Laboratories) were used at a 1:5,000 dilution, and proteins were detected with Pierce SuperSignal West Dura substrate (Pierce Biotechnology).
Anti-phosphoSer51-specific rabbit monoclonal antibody (Abcam, ab32157) was used at a 1:500 dilution, and proteins were detected with the ReliBlot secondary antibody system from Bethyl Laboratories and Pierce SuperSignal West Dura substrate (Pierce Biotechnology).
Microarray analysis of miRNA.
An in-house miRNA microarray system was used to survey miRNA expression on a genome-wide scale (22). Microarray analyses were performed on three biological replicates for every time point and polysome population. We focused on signals from the only mammalian (human, mouse, and rat) miRNA probes on the custom microarrays, and every miRNA probe was treated as a separate miRNA, without combining homologous probes from the same miRNA family or from different species. After background subtraction, the intensities of miRNA probes were normalized to that of the internal 28S rRNA probe or of the 5SN1 small nucleolar RNA (snoRNA) probe included in the probe set. The resulting relative miRNA expression levels were then used for comparisons across slides/samples. Because normalizations to 28S rRNA and to 5SN1 snoRNA led to identical conclusions, only results from the 28S rRNA are presented here. When the same quantities of RNAs from different polysome populations were analyzed, their 28S rRNA signals were similar in strength.
Evaluation of miRNA by Northern blot.
Approximately 20 μg of RNAs from each polysomal fraction was run on a 7 M urea-10% polyacrylamide gel and transferred to a Hybond-N+ membrane (GE Healthcare) for Northern blot detection of miRNA (22). The membrane was then probed with an end-labeled oligonucleotide complementary to the miRNA of interest and analyzed afterwards with a PhosphorImager (GE Healthcare). The same membrane was reused to detect different miRNAs; it was stripped of existing signals before being hybridized to the next miRNA probe. Sequences of the oligonucleotides end-labeled for probes were let-7a: 5′-AACTATACAACCTACTACCTCA-3′; miR-21: 5′-GCTAGTCAACATCAGTCTGATA-3′ (42); and miR-451: 5′-AAACTCAGTAATGGTAACGGTTT-3′.
Densitometry and statistics.
The relative intensities of the ethidium bromide-stained PCR products after agarose gel electrophoresis and protein bands detected by Western blot were analyzed by densitometry with a Bio-Rad model GS-700 imaging densitometer. The percent change in transcript levels relative to nonresected liver polysomes was calculated after normalization to the rRNA 18S PCR product control. Statistical analysis was performed with GraphPad InStat version 3.1 for OSX (GraphPad Software, San Diego, CA) for the ANOVA and Bonferroni's multiple comparison tests used. Values of P < 0.05 were considered significant.
RESULTS
Polysome-associated RNA profiles during liver regeneration.
We initially examined the RNA abundance in each of the three discrete polysome populations from quiescent and regenerating liver. Purity of the isolated polysome fractions was assessed by lactate dehydrogenase activity and vimentin, as markers for free and membrane fractions, respectively (40). As predicted, the bulk of enzymatic activity was released in the initial cell lysis for the free population irrespective of the time after PH (Table 1). Minimal activity was observed in the membrane fraction, while the cytoskeletal fraction contained between 6% and 10% of total activity. Western blot analysis showed that vimentin was significantly enriched in the membrane fraction, present at a low level in the cytoskeletal fraction, and undetectable in the free fraction (data not shown).
Table 1.
Lactate dehydrogenase activity in polysomal fractions
| % LDH Activity |
|||
|---|---|---|---|
| Time After PH, h | Free | Cytoskeletal | Membrane |
| 0 | 86.5±8.1 | 10.6±6.5 | 2.9±1.9 |
| 3 | 91.9±2.6 | 6.0±1.9 | 2.1±0.8 |
| 6 | 86.4±2.4 | 10.7±2.2 | 2.9±0.9 |
| 24 | 89.7±4.4 | 6.7±3.0 | 3.5±1.5 |
Values are mean ± SD % of total lactate dehydrogenase (LDH) activity in each of the 3 polysomal fractions before their block gradient isolation. Tissue was harvested for isolation of the polysomal fractions as outlined in materials and methods at indicated times after 70% partial hepatectomy (PH).
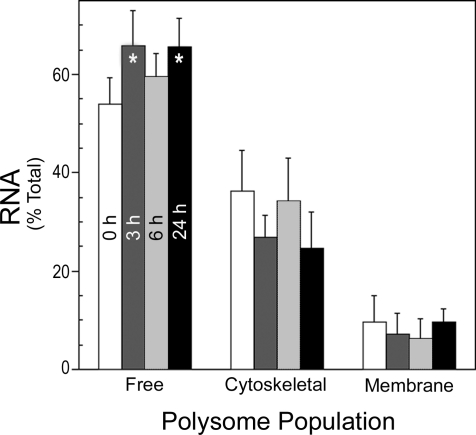
The polysomal fractions were further purified by sucrose block gradient ultracentrifugation to separate unbound ribosomes and RNAs before total RNA extraction. The percent total RNA isolated from each polysome population at 0, 3, 6, and 24 h after PH is shown in Fig. 1. The data showed that >50% of the polysome-associated RNA was present in the free fraction, and this percentage increased during liver regeneration. The cytoskeleton-bound polysomes contained ∼35% of the total polysome RNA in quiescent liver but decreased ∼10% at 3 and 24 h after PH. The membrane-bound polysomes contained <10% of the total RNA associated with polysomes, and this decreased slightly at 3 and 6 h, returning to baseline levels by 24 h. Together the data indicated that the isolation/fractionation procedure yielded discrete populations with minimal cross-contamination of the fractions. Moreover, the percent RNA in the three polysome populations differed dramatically and changed significantly during liver regeneration.
Fig. 1.
Flux of RNA through the different polysome populations. Liver tissue was processed as described in materials and methods to generate extracts containing free and cytoskeleton- and membrane-bound polysomes. Mean ± SD % of total RNA isolated in each of the fractions at the indicated times after 70% partial hepatectomy (PH) is shown. *P < 0.05 from 0 h free polysome %.
Quantification of c-myc and p53 transcript levels in the three polysome populations.
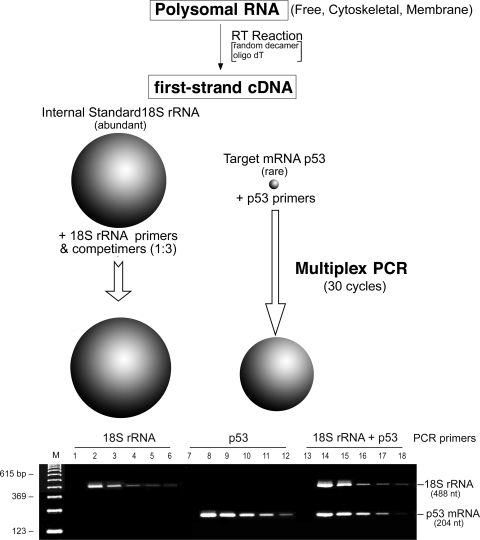
We next determined the abundance of c-myc and p53 transcript levels in free and cytoskeleton- and membrane-bound populations during regeneration after PH. We chose to use 18S rRNA as the internal control because of its invariant expression. However, because of its high-level expression compared with c-myc and p53, we were required to use Competimer specially modified primers that could not be extended for RT-PCR. By adjusting the ratio of Competimers to extendable 18S rRNA primers, the 18S rRNA signal could be attenuated to the appropriate level of the target message by modulating the efficiency of amplification of the 18S PCR product (Fig. 2, top). Using this methodology, we first optimized the RT-PCR for the 18S rRNA primer-to-Competimer ratio and target transcript individually and then in multiplex PCR with cDNA amounts from 10 to 250 ng (Fig. 2, bottom). Analysis of the gels by densitometry indicated that a primer-to-Competimer ratio of 1:3 in 30-cycle multiplex PCR provided linear amplification over a 10–250 ng cDNA input range for 18S rRNA, p53, and c-myc.
Fig. 2.
Validation of 18S rRNA and Competimers for normalization. The invariant expression of 18S ribosomal RNA makes it an ideal internal control for quantitative RNA analysis. However, because of its high level relative to the target mRNA, it is necessary to attenuate amplification of the 18S rRNA to the level anticipated from the target message. Top: to achieve this, with QuantumRNA Competimers, which are specially modified primers that cannot be extended, adjusting the ratio of functional 18S rRNA primers to Competimers modulates the efficiency of amplification of the 18S PCR product from the first-strand cDNA template. The linear range of 10–250 ng RNA as template was used for initial RT-PCR reactions with 18S rRNA specific primers (P) and 18S rRNA Competimers (C) and the 30-cycle amplification protocol described in materials and methods. Bottom: representative agarose gel of the PCR products obtained with a P-to-C ratio of 1:3 visualized by ethidium bromide staining and ultraviolet light. Predicted size of the 18S rRNA is shown at left; sizes of the DNA markers (M) are indicated at right. Lanes 1, 7, 13, no RT; lanes 2, 8, 14, cDNA template from RT-PCR using 250 ng RNA; lanes 3, 9, 15, 125 ng RNA; lanes 4, 10, 16, 75 ng RNA; lanes 5, 11, 17, 25 ng RNA; lanes 6, 12, 18, 10 ng RNA.
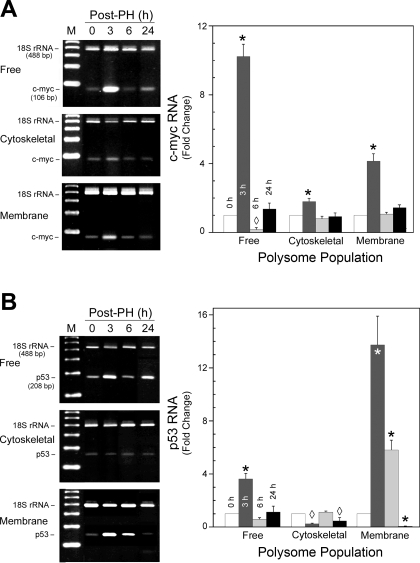
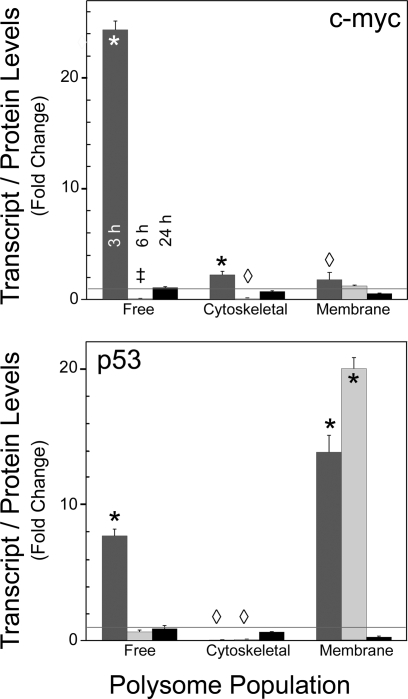
With these optimized conditions, RNA was isolated from the different polysome populations for Competimer RT-PCR using the 18S rRNA as the internal standard. The results for c-myc indicated that at 3 h after PH all polysome fractions showed a significant increase in free (10-fold) and membrane (4-fold)- and cytoskeleton (2-fold)-bound transcript levels over nonresected liver (Fig. 3A). By 6 h, only the free fraction showed a significant decrease to ∼10% of time 0 levels; by 24 h all had returned to basal values. The transcripts for p53 were also significantly elevated relative to nonresected liver in the free (4-fold) and membrane (13-fold) populations at 3 h after PH, while its abundance decreased to ∼10% of baseline in the cytoskeletal fraction (Fig. 3B). At 6 h, only the membrane fraction remained significantly elevated, with levels greater than sixfold over time 0, while the free and cytoskeletal fractions returned to near baseline values. Interestingly, at 24 h, the membrane-bound and cytoskeletal fractions had levels significantly lower than time 0, while the free fraction remained unchanged. In contrast, total RNA from the polysome fractions isolated with buffers containing 25 mM EDTA gave no c-myc- or p53-specific amplicons and substantially less (∼10%) 18S rRNA internal standard by RT-PCR under the same conditions (data not shown). This was consistent with the substantial reduction (P < 0.001) in RNA isolated from the EDTA-treated samples. While it is possible that the low levels of 18S rRNA resulted from aggregated ribosomal subunits, it is conceivable that some heavily ribosome-loaded transcripts were incompletely disrupted or failed to entirely dissociate the 40S subunits from the transcript. Together, these data demonstrate that the c-myc and p53 transcripts are associated with all three polysome populations in rat liver. The abundance of the transcript in a particular polysome population undergoes dynamic changes during liver regeneration.
Fig. 3.
Flux of c-myc and p53 mRNAs through the 3 different polysome populations during rat liver regeneration. Representative agarose gels and densitometric analyses of the PCR products obtained with Competimer PCR for c-myc (A) and p53 (B) visualized by ethidium bromide staining and ultraviolet light are shown. Predicted size and identity of the 18S rRNA and transcript-specific amplicon are shown at left, with the specific polysome population. Fold change normalized to the 18S rRNA signal relative to 0 time is shown in the graphs and represents means ± SD from ≥3 animals. *P < 0.001, ◊P < 0.05 compared with time 0.
Polysome protein levels.
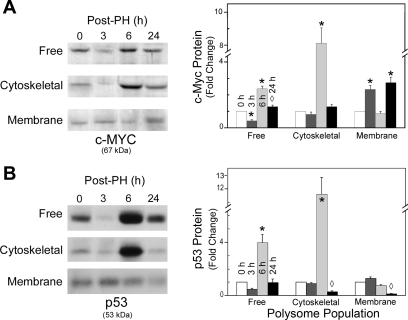
Western blot analysis for c-myc and p53 were also performed on the discrete polysome populations and correlated with the respective mRNA profiles. In contrast to the elevated c-myc transcript levels at 3 h, the free and cytoskeleton-bound polysomes had no significant change in levels of c-myc protein relative to time 0 (Fig. 4A). However, the increased transcript levels in the membrane fraction correlated with increased c-myc protein (P < 0.001). After 6 h, c-myc mRNA levels had returned to basal levels or below, but substantial increases (P < 0.001) in c-myc protein in these two fractions were observed, while the membrane-bound polysomes exhibited basal levels of c-myc protein. By 24 h, only the membrane-bound polysome fraction had significantly elevated levels of c-myc protein (P < 0.001) relative to time 0. The discordance in elevation of transcript and protein levels in a polysome population was even more pronounced for p53 than for c-myc. Although 4- to 14-fold elevations in transcript levels for p53 were noted at 3 h in the free and membrane-bound polysomes, no corresponding change in protein levels was observed (Fig. 4B). At 6 h after PH, although transcript levels in the cytoskeleton-bound and free populations did not differ from time 0, there were dramatic increases (P < 0.001) in nascent p53 protein levels, which returned to basal by 24 h. The approximately sixfold increase in transcript levels at 6 h did not show a corresponding increase of protein in the membrane-bound fraction. However, the decrease in transcript at 24 h relative to time 0 was associated with decreased p53 protein abundance.
Fig. 4.
Levels of c-myc and p53 nascent proteins detected in the different polysome populations. Representative Western blots of c-myc (A) and p53 (B) detected with anti-c-myc or p53 primary antibodies and horseradish peroxidase-conjugated secondary antibodies by enhanced chemiluminescence as described in materials and methods are shown. Protein and predicted size are indicated at bottom; polysome population and time after PH are shown at left and above the Western blots, respectively. Densitometric analyses of the c-myc (A) and p53 (B) protein levels are shown at right of their respective Western blots. Fold change relative to time 0 in the graphs represents the mean ± SD from ≥3 animals. *P < 0.001, ◊P < 0.05 compared with time 0.
The polysome association of the c-myc and p53 proteins was confirmed by Western blot analysis of total protein from the three populations isolated in the presence or absence of 25 mM EDTA. While the ribosome protein L26 was readily detected in total protein from all three populations, p53 and c-myc were not detected in the protein from polysomes isolated with EDTA present. Thus, although the proteins detected by Western blot analysis appeared to be “full length,” their association with the free, cytoskeleton-bound, or membrane-bound populations was dependent on intact polysomes, e.g., ribosomes engaged with mRNAs. Finally, Western blot analysis was performed for the endoplasmic reticulum marker calnexin to investigate whether other large subcellular components also were brought down with the polysomes. No calnexin was detected in the membrane-bound fraction (data not shown), indicating that the association observed for c-myc and p53 proteins was polysome dependent.
Protein and transcript levels are uncoupled during liver regeneration.
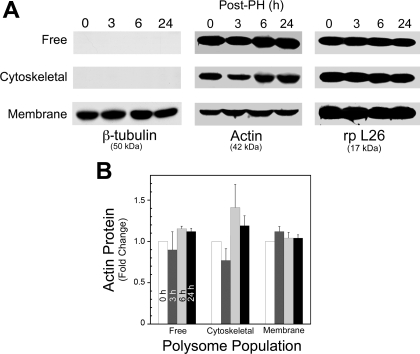
To establish whether these changes in protein levels were unique to p53 and c-myc in the different polysome fractions, actin and β-tubulin levels were also characterized by Western blot. The results (Fig. 5A) indicted that β-tubulin protein was only associated with the membrane-bound fraction and its concentration did not change during liver regeneration. With an alternate anti-tubulin antibody that detects both isoforms, invariant tubulin levels were detected in the free polysome fraction as well (data not shown). In contrast, actin protein levels in the free and cytoskeleton-bound polysome populations were modulated after PH (Fig. 5A), while little change was observed in the membrane-bound population. However, the changes observed in the free and cytoskeleton-associated populations were neither as extensive nor significant (Fig. 5B) in contrast to that observed for c-myc and p53 (Fig. 4, A and B, respectively). Ribosomal protein L26 analysis by Western blot indicated that the abundance per microgram of total polysomal protein in the free, cytoskeletal, and membrane-bound fractions differed but remained constant within each fraction at the indicated times after PH (Fig. 5A). This suggested that the dramatic modulation of protein levels in the different polysome populations during liver regeneration was transcript specific. Our results indicated a significant uncoupling of c-myc and p53 transcript and protein level in the different polysome populations during the first 24 h of rat liver regeneration (Fig. 6).
Fig. 5.
Western blot analysis of β-tubulin, actin, and ribosomal L26 proteins in the different polysomes. Actin and β-tubulin, commonly used controls for analysis of nascent protein levels were determined in the 3 different block gradient-purified polysome populations after PH as described in materials and methods. A: Western blot analysis of polysome-associated β-tubulin and actin nascent proteins and the large ribosome subunit protein rp L26. This shows that β-tubulin is associated with a single polysome population and is not modulated after PH and that the % of ribosomes present per microgram of total protein is different in the 3 populations. Protein and predicted size are indicated at bottom. Time after PH and polysome population are shown at top and at left of Western blots, respectively. B: flux of actin protein expression in the different polysome populations after PH relative to time 0. Data are means ± SD from ≥3 animals.
Fig. 6.
Modulation of transcript-to-protein ratios in the different populations. Changes in the ratios observed in the different polysome populations for c-myc and p53 are shown at top and bottom, respectively; time 0 ratios between transcript and nascent protein levels in arbitrary densitometric units are set to 1. A significant deviation from unity (horizontal line) indicates that the ratio between steady-state levels of transcripts and protein changed in the polysome population relative to quiescent liver. *P < 0.001, ‡P < 0.01, ◊P < 0.05 compared with time 0.
Association of miRNAs with the three discrete cellular polysome populations.
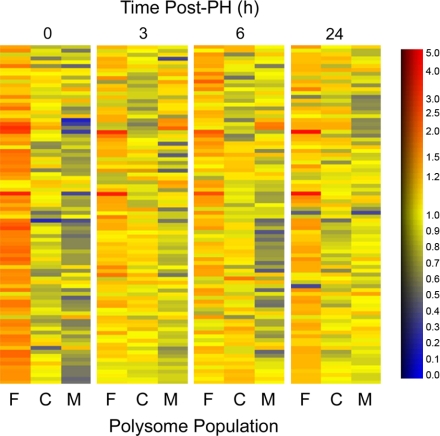
A primary function of miRNAs in mammalian cells is to regulate translational activity of mRNAs. Thus we investigated the miRNA profiles associated with the free and cytoskeleton- and membrane-bound polysomes in both quiescent and regenerating rat liver. The results are presented as a heat map for the miRNAs with the highest signals in the polysomal populations (Fig. 7).
Fig. 7.
Distribution of 85 distinct mammalian microRNAs (miRNAs) in regenerating liver. miRNA probes that gave the strongest hybridization signals in all 3 polysome populations at 0, 3, 6, and 24 h after PH are shown. At time 0, the free (F) polysome population contained the strongest normalized hybridization signals for practically all the miRNA probes. Relative abundance of individual miRNAs in different polysome populations is presented as heat maps. At 0 h, the free polysome population (far left) is dominated by orange to red colors, which signify high relative abundance, while the cytoskeletal (C) and membrane-bound (M) populations are colored mostly by blue and yellow, indicating lower expression levels. The scale is shown at right, with the relative abundance in arbitrary units.
Practically all miRNAs detected in the total rat liver RNA were also found in the free and cytoskeleton- and membrane-bound polysome populations (data not shown). At 0 h, the free polysome population contained the strongest normalized hybridization signals for practically all the miRNA probes. Although individual miRNAs varied in their polysome distribution, in general relative miRNA levels in the cytoskeleton- and membrane-bound populations were ∼60% and 30–40% of those in the free polysomes, respectively (Tables 2–4). At time 0, the free polysome population (Fig. 7, far left) is dominated by orange to red colors, which signify high relative abundance, while the other two populations are colored by mostly blue and yellow, indicating lower expression levels.
Table 2.
miRNAs that increase relative abundance in C and M polysome populations from 3 to 24 h after PH
| Time After PH, h |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
3 |
6 |
24 |
|||||||||
| miRNA | F | C | M | F | C | M | F | C | M | F | C | M |
| hsa-let-7a|rno-let-7a | 100 | 68 | 37 | 100 | 81 | 98 | 100 | 89 | 108 | 100 | 69 | 67 |
| hsa-let-7b|mmu-let-7b|rno-let-7b | 100 | 55 | 29 | 100 | 93 | 101 | 100 | 105 | 128 | 100 | 74 | 66 |
| hsa-let-7c|mmu-let-7c|rno-let-7c | 100 | 61 | 35 | 100 | 85 | 89 | 100 | 98 | 118 | 100 | 72 | 59 |
| hsa-let-7d|mmu-let-7d|rno-let-7d | 100 | 57 | 32 | 100 | 85 | 88 | 100 | 90 | 115 | 100 | 76 | 74 |
| hsa-let-7e|mmu-let-7e|rno-let-7e | 100 | 69 | 35 | 100 | 97 | 100 | 100 | 102 | 115 | 100 | 71 | 67 |
| hsa-let-7f|rno-let-7f | 100 | 56 | 32 | 100 | 89 | 101 | 100 | 80 | 98 | 100 | 65 | 67 |
| hsa-let-7 g|mmu-let-7 g | 100 | 56 | 34 | 100 | 88 | 108 | 100 | 82 | 97 | 100 | 62 | 63 |
| hsa-miR-100|mmu-miR-100|rno-miR-100 | 100 | 43 | 31 | 100 | 63 | 93 | 100 | 60 | 111 | 100 | 55 | 60 |
| hsa-miR-101|mmu-miR-101a|rno-miR-101a | 100 | 40 | 36 | 100 | 62 | 89 | 100 | 44 | 73 | 100 | 54 | 68 |
| hsa-miR-122a|mmu-miR-122a|rno-miR-122a | 100 | 69 | 54 | 100 | 71 | 88 | 100 | 108 | 123 | 100 | 80 | 91 |
| hsa-miR-125b|mmu-miR-125b|rno-miR-125b | 100 | 57 | 41 | 100 | 91 | 114 | 100 | 98 | 129 | 100 | 76 | 82 |
| hsa-miR-126|mmu-miR-126-3p|rno-miR-126 | 100 | 45 | 31 | 100 | 85 | 102 | 100 | 72 | 112 | 100 | 54 | 69 |
| hsa-miR-143|mmu-miR-143|rno-miR-143 | 100 | 75 | 55 | 100 | 98 | 118 | 100 | 114 | 170 | 100 | 63 | 71 |
| hsa-miR-148a|mmu-miR-148a | 100 | 42 | 31 | 100 | 79 | 106 | 100 | 84 | 117 | 100 | 64 | 70 |
| hsa-miR-16|mmu-miR-16|rno-miR-16 | 100 | 54 | 43 | 100 | 71 | 104 | 100 | 97 | 137 | 100 | 65 | 69 |
| hsa-miR-191|mmu-miR-191|rno-miR-191 | 100 | 70 | 63 | 100 | 85 | 106 | 100 | 115 | 154 | 100 | 77 | 86 |
| hsa-miR-192|rno-miR-192 | 100 | 42 | 33 | 100 | 58 | 82 | 100 | 46 | 86 | 100 | 52 | 69 |
| hsa-miR-194|mmu-miR-194|rno-miR-194 | 100 | 46 | 32 | 100 | 62 | 79 | 100 | 41 | 67 | 100 | 51 | 60 |
| hsa-miR-195|mmu-miR-195|rno-miR-195 | 100 | 61 | 35 | 100 | 80 | 96 | 100 | 87 | 137 | 100 | 57 | 57 |
| hsa-miR-206|mmu-miR-206|rno-miR-206 | 100 | 53 | 48 | 100 | 120 | 137 | 100 | 85 | 259 | 100 | 98 | 108 |
| hsa-miR-21|mmu-miR-21|rno-miR-21 | 100 | 37 | 37 | 100 | 57 | 80 | 100 | 46 | 73 | 100 | 45 | 69 |
| hsa-miR-215 | 100 | 42 | 33 | 100 | 62 | 84 | 100 | 48 | 91 | 100 | 54 | 64 |
| hsa-miR-22|mmu-miR-22|rno-miR-22 | 100 | 44 | 28 | 100 | 67 | 104 | 100 | 51 | 73 | 100 | 49 | 62 |
| hsa-miR-23a|mmu-miR-23a|rno-miR-23a | 100 | 48 | 40 | 100 | 65 | 92 | 100 | 74 | 122 | 100 | 61 | 70 |
| hsa-miR-23b|mmu-miR-23b|rno-miR-23b | 100 | 41 | 35 | 100 | 76 | 89 | 100 | 67 | 108 | 100 | 62 | 69 |
| hsa-miR-26a|mmu-miR-26a|rno-miR-26a | 100 | 41 | 27 | 100 | 69 | 96 | 100 | 55 | 92 | 100 | 57 | 62 |
| hsa-miR-26b|mmu-miR-26b|rno-miR-26b | 100 | 49 | 33 | 100 | 68 | 89 | 100 | 57 | 83 | 100 | 61 | 75 |
| hsa-miR-29a|mmu-miR-29a|rno-miR-29a | 100 | 56 | 38 | 100 | 86 | 100 | 100 | 68 | 121 | 100 | 73 | 91 |
| hsa-miR-29b|mmu-miR-29b|rno-miR-29b | 100 | 42 | 28 | 100 | 85 | 116 | 100 | 62 | 93 | 100 | 64 | 80 |
| hsa-miR-29c|mmu-miR-29c|rno-miR-29c | 100 | 50 | 35 | 100 | 102 | 95 | 100 | 62 | 112 | 100 | 76 | 96 |
| hsa-miR-30b|mmu-miR-30b|rno-miR-30b | 100 | 31 | 22 | 100 | 64 | 86 | 100 | 52 | 68 | 100 | 46 | 51 |
| hsa-miR-30c|mmu-miR-30c|rno-miR-30c | 100 | 35 | 23 | 100 | 60 | 79 | 100 | 49 | 86 | 100 | 48 | 58 |
| hsa-miR-363*|rno-miR-363-5p | 100 | 44 | 48 | 100 | 42 | 112 | 100 | 107 | 119 | 100 | 77 | 41 |
| hsa-miR-422a | 100 | 50 | 36 | 100 | 66 | 52 | 100 | 50 | 61 | 100 | 50 | 57 |
| hsa-miR-422b|mmu-miR-422b | 100 | 61 | 45 | 100 | 79 | 66 | 100 | 50 | 63 | 100 | 61 | 62 |
| hsa-miR-765 | 100 | 66 | 78 | 100 | 135 | 162 | 100 | 147 | 141 | 100 | 110 | 132 |
| hsa-miR-768-5p | 100 | 64 | 48 | 100 | 137 | 134 | 100 | 137 | 113 | 100 | 99 | 117 |
| hsa-miR-98|mmu-miR-98|rno-miR-98 | 100 | 65 | 40 | 100 | 86 | 100 | 100 | 97 | 117 | 100 | 59 | 51 |
| hsa-miR-99a|rno-miR-99a | 100 | 55 | 55 | 100 | 77 | 103 | 100 | 69 | 108 | 100 | 61 | 99 |
| mmu-let-7f | 100 | 57 | 35 | 100 | 105 | 99 | 100 | 81 | 108 | 100 | 74 | 75 |
| mmu-miR-106a | 100 | 39 | 40 | 100 | 33 | 77 | 100 | 85 | 80 | 100 | 97 | 65 |
| mmu-miR-192 | 100 | 37 | 28 | 100 | 57 | 92 | 100 | 49 | 85 | 100 | 54 | 70 |
| mmu-miR-466 | 100 | 40 | 20 | 100 | 286 | 84 | 100 | 239 | 96 | 100 | 94 | 40 |
| mmu-miR-467a | 100 | 46 | 11 | 100 | 204 | 75 | 100 | 195 | 91 | 100 | 92 | 42 |
| mmu-miR-467b | 100 | 66 | 12 | 100 | 130 | 49 | 100 | 124 | 54 | 100 | 90 | 42 |
| mmu-miR-690 | 100 | 44 | 29 | 100 | 85 | 53 | 100 | 68 | 76 | 100 | 94 | 52 |
| mmu-miR-691 | 100 | 67 | 56 | 100 | 127 | 99 | 100 | 114 | 121 | 100 | 93 | 78 |
| mmu-miR-101b|rno-miR-101b | 100 | 36 | 27 | 100 | 62 | 81 | 100 | 41 | 66 | 100 | 55 | 73 |
| rno-miR-352 | 100 | 52 | 30 | 100 | 93 | 112 | 100 | 97 | 132 | 100 | 86 | 71 |
Values are the averages of 3 sets of biological replicates for each polysome population and time point. MicroRNAs (miRNAs) are from the 85 probes that gave the highest hybridization signals on the microarray heat map shown in Fig. 7. Names of miRNAs are from the Invitrogen probe set, but only the hsa (human), mmu (mouse), and rno (rat) miRNAs are listed. Polysomes were isolated from regenerating liver at indicated times after PH. F, free polysome-associated miRNAs (level of miRNA probe hybridized in this population was assigned a value of 100 at each time point); C, cytoskeleton-bound polysome miRNAs as % of free polysome miRNAs at the same times after PH; M, membrane-bound polysome miRNAs as % of free polysome miRNA levels at the same times after PH.
Table 3.
miRNAs whose relative abundance in free polysome population is increased after PH
| Time After PH, h |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
3 |
6 |
24 |
|||||||||
| miRNA | F | C | M | F | C | M | F | C | M | F | C | M |
| hsa-miR-184|mmu-miR-184|rno-miR-184 | 100 | 91 | 160 | 100 | 100 | 73 | 100 | 60 | 42 | 100 | 97 | 78 |
| hsa-miR-214|mmu-miR-214|rno-miR-214 | 100 | 78 | 105 | 100 | 59 | 51 | 100 | 49 | 48 | 100 | 89 | 74 |
| mmu-miR-705 | 100 | 101 | 125 | 100 | 136 | 77 | 100 | 87 | 64 | 100 | 74 | 57 |
| mmu-miR-711 | 100 | 79 | 131 | 100 | 83 | 63 | 100 | 58 | 65 | 100 | 52 | 49 |
Values are averages of 3 sets of biological replicates for each polysome population and time point. miRNAs are from the 85 probes that gave the highest hybridization signals on the microarray heat map shown in Fig. 7. Names of miRNAs are from the Invitrogen probe set, but only the hsa (human), mmu (mouse), and rno (rat) miRNAs are listed. Polysomes were isolated from regenerating liver at indicated times after PH. F, free polysome-associated miRNAs (level of miRNA probe hybridized in this population was assigned a value of 100 at each time point); C, cytoskeleton-bound polysome miRNAs as % of free polysome miRNAs at the same times after PH; M, membrane-bound polysome miRNAs as % of free polysome miRNA levels at the same times after PH.
Table 4.
miRNAs that maintain constant distribution in all three polysome populations after PH
| Time After PH, h |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 |
3 |
6 |
24 |
|||||||||
| miRNA | F | C | M | F | C | M | F | C | M | F | C | M |
| hsa-miR-106a | 100 | 46 | 114 | 100 | 77 | 77 | 100 | 87 | 69 | 100 | 79 | 64 |
| hsa-miR-130a|mmu-miR-130a|rno-miR-130a | 100 | 57 | 72 | 100 | 60 | 69 | 100 | 60 | 86 | 100 | 112 | 100 |
| hsa-miR-185|mmu-miR-185|rno-miR-185 | 100 | 70 | 71 | 100 | 109 | 102 | 100 | 155 | 146 | 100 | 67 | 65 |
| hsa-miR-198 | 100 | 90 | 100 | 100 | 113 | 94 | 100 | 72 | 76 | 100 | 100 | 93 |
| hsa-miR-19b|mmu-miR-19b|rno-miR-19b | 100 | 60 | 57 | 100 | 75 | 61 | 100 | 38 | 58 | 100 | 40 | 52 |
| hsa-miR-202 | 100 | 63 | 84 | 100 | 95 | 84 | 100 | 83 | 99 | 100 | 114 | 133 |
| hsa-miR-205|mmu-miR-205|rno-miR-205 | 100 | 77 | 77 | 100 | 108 | 104 | 100 | 154 | 173 | 100 | 58 | 90 |
| hsa-miR-20a|mmu-miR-20a|rno-miR-20a | 100 | 40 | 63 | 100 | 49 | 65 | 100 | 83 | 66 | 100 | 110 | 75 |
| hsa-miR-30d|mmu-miR-30d|rno-miR-30d | 100 | 55 | 50 | 100 | 55 | 65 | 100 | 52 | 72 | 100 | 57 | 67 |
| hsa-miR-320|mmu-miR-320|rno-miR-320 | 100 | 77 | 121 | 100 | 56 | 69 | 100 | 94 | 89 | 100 | 118 | 111 |
| hsa-miR-335|mmu-miR-335|rno-miR-335 | 100 | 59 | 58 | 100 | 95 | 96 | 100 | 53 | 55 | 100 | 82 | 104 |
| hsa-miR-373* | 100 | 81 | 51 | 100 | 82 | 57 | 100 | 72 | 53 | 100 | 84 | 51 |
| hsa-miR-451 | 100 | 25 | 10 | 100 | 24 | 19 | 100 | 24 | 31 | 100 | 19 | 14 |
| hsa-miR-494 | 100 | 85 | 101 | 100 | 53 | 71 | 100 | 73 | 85 | 100 | 82 | 83 |
| hsa-miR-638 | 100 | 119 | 93 | 100 | 128 | 87 | 100 | 56 | 59 | 100 | 81 | 66 |
| hsa-miR-663 | 100 | 119 | 111 | 100 | 207 | 101 | 100 | 59 | 60 | 100 | 112 | 125 |
| mmu-miR-202 | 100 | 72 | 117 | 100 | 90 | 78 | 100 | 83 | 86 | 100 | 96 | 104 |
| mmu-miR-20b | 100 | 39 | 44 | 100 | 41 | 80 | 100 | 81 | 69 | 100 | 91 | 79 |
| mmu-miR-290|rno-miR-290 | 100 | 74 | 64 | 100 | 66 | 83 | 100 | 76 | 35 | 100 | 105 | 65 |
| mmu-miR-292-5p|rno-miR-292-5p | 100 | 64 | 44 | 100 | 55 | 42 | 100 | 47 | 35 | 100 | 57 | 52 |
| mmu-miR-451|rno-miR-451 | 100 | 28 | 12 | 100 | 23 | 21 | 100 | 27 | 33 | 100 | 16 | 14 |
| mmu-miR-494|rno-miR-494 | 100 | 84 | 125 | 100 | 55 | 74 | 100 | 68 | 69 | 100 | 101 | 106 |
| mmu-miR-667 | 100 | 101 | 64 | 100 | 103 | 71 | 100 | 89 | 65 | 100 | 121 | 63 |
| mmu-miR-706 | 100 | 51 | 51 | 100 | 65 | 65 | 100 | 73 | 78 | 100 | 104 | 86 |
| mmu-miR-709 | 100 | 81 | 83 | 100 | 128 | 115 | 100 | 126 | 93 | 100 | 96 | 98 |
| mmu-miR-721 | 100 | 88 | 67 | 100 | 95 | 60 | 100 | 60 | 56 | 100 | 105 | 56 |
| mmu-miR-744 | 100 | 124 | 122 | 100 | 219 | 125 | 100 | 37 | 51 | 100 | 69 | 83 |
| mmu-miR-762 | 100 | 104 | 109 | 100 | 150 | 83 | 100 | 53 | 44 | 100 | 79 | 74 |
| mmu-miR-763 | 100 | 116 | 103 | 100 | 109 | 84 | 100 | 96 | 87 | 100 | 102 | 95 |
| rno-miR-140* | 100 | 83 | 76 | 100 | 85 | 76 | 100 | 81 | 81 | 100 | 92 | 78 |
| rno-miR-327 | 100 | 109 | 103 | 100 | 106 | 73 | 100 | 100 | 65 | 100 | 100 | 75 |
| rno-miR-422b | 100 | 53 | 60 | 100 | 78 | 86 | 100 | 80 | 97 | 100 | 67 | 65 |
Values are averages of 3 sets of biological replicates for each polysome population and time point. miRNAs are from the 85 probes that gave the highest hybridization signals on the microarray heat map shown in Fig. 7. Names of miRNAs are from the Invitrogen probe set, but only hsa (human), mmu (mouse), and rno (rat) miRNAs are listed. Polysomes were isolated from regenerating liver at indicated times after PH. F, free polysome-associated miRNAs (level of miRNA probe hybridized in this population was assigned a value of 100 at each time point); C, cytoskeleton-bound polysome miRNAs as % of free polysome miRNAs at the same times after PH; M, membrane-bound polysome miRNAs as % of free polysome miRNA levels at the same times after PH.
At 3, 6, and 24 h after PH, the combined relative expression levels of miRNAs in the three polysome populations changed very little (data not shown), yet the partition of many miRNAs to the populations changed dramatically. The free polysomes still had the highest relative hybridization signals for most miRNA probes, but the cytoskeleton-bound polysomes and, especially, the membrane-bound polysomes contained increased levels of miRNAs relative to the free polysomes. Of the 85 miRNAs (probes) shown in Fig. 7, 49 clearly exhibited such a change in distribution (Table 2). Four miRNA probes showed an increase in signal partition to the free polysomes compared with the nonresected liver (miR-184, 214, mmu-miR-705, and mmu-miR-711; Table 3). The remaining miRNAs did not change their polysome distribution pattern appreciably (Table 4).
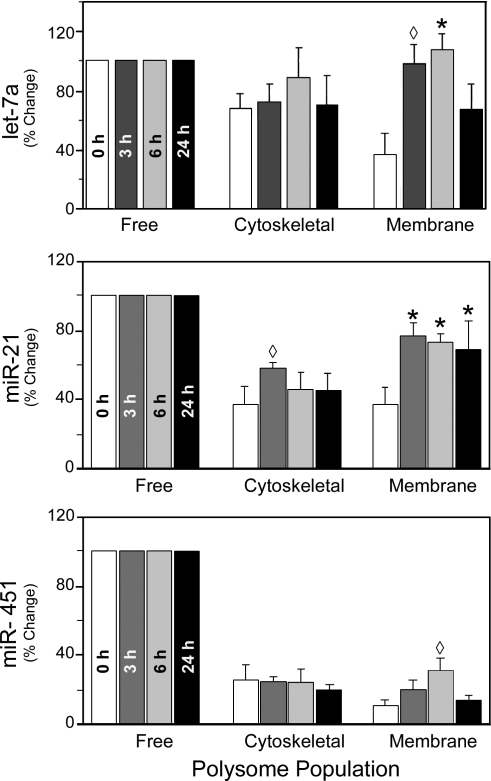
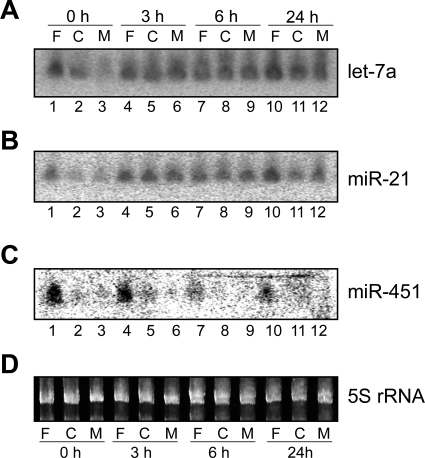
To illustrate the changes in miRNA polysome distribution after PH, three examples are presented in Fig. 8. As shown in Table 2, the average let-7a expression levels at the membrane-associated polysome population relative to that of the free polysome population increased from 37% at 0 h to 98%, 108%, and 67% at 3, 6, and 24 h, respectively. The relative levels of let-7a in the cytoskeleton-associated polysome population did not change significantly. This may be due to the fact that at time 0, average let-7a distribution to the cytoskeleton-associated polysomes was already at a high level of 68% relative to its distribution to the free polysomes. Northern blot analysis (Fig. 9) using RNA samples from one set of rats confirmed that let-7a levels in the membrane-associated polysome population indeed increased after PH (Fig. 9A, compare lanes 6, 9, and 12 with lane 3). These results also demonstrated that our miRNA microarray platform provided a reliable way to measure miRNA expression levels. While it may be difficult to distinguish between different let-7 miRNA family members, both let-7s and miR-98 behaved similarly in our microarray studies (Table 2). miR-21 is another miRNA that increased its relative abundance in the cytoskeleton- and membrane-bound polysome populations after PH. For example, its average levels in the membrane-bound population relative to that of the free polysome population increased from 37% at 0 h to 80%, 73%, and 69% at 3, 6, and 24 h, respectively (Fig. 8). Again, Northern blotting confirmed such changes (Fig. 9B, compare lanes 5, 6, 8, 9, 11, and 12 with lanes 2 and 3). We performed additional Northern blot analyses on miR-195 and miR-215, which had the same distribution pattern as let-7a and miR-21 (Table 2), and the results confirmed the microarray studies (data not shown).
Fig. 8.
Distribution of specific miRNAs in free and cytoskeleton- and membrane-bound polysome populations in regenerating liver. A: relative let-7a expression levels in free and cytoskeleton- and membrane-bound polysomes at 0, 3, 6, and 24 h after PH were determined by microarray studies. Relative let-7a expression in the free polysomes was set at 100% for each time point, and the same polysome populations are grouped together for comparisons. B: miR-21 distribution in the 3 polysome populations at 0, 3, 6 and 24 h after PH. C: levels of miR-451 in the polysome populations after PH. Data are from signals of the mmu-miR-451|rno-miR-451|dre-miR-451|xtr-miR-451 probe, while the hsa-miR-451|gga-miR-451 probe yielded virtually identical results (Table 1). Means ± SD of 3 sets of data for each polysome population and time point are shown. *P < 0.001, ‡P < 0.01, ◊P < 0.05 compared with time 0 in the same polysome population.
Fig. 9.
Northern blot analyses of selected mature miRNAs. Northern blotting was performed as described in materials and methods with probes for let-7a (A), miR-21 (B), or miR-451 (C). D: ethidium bromide-stained gel before transfer, showing approximately equal amounts of 5S rRNA in each lane. Lanes 1, 4, 7, and 10, RNAs isolated from free polysome fractions; lanes 2, 5, 8, and 11, cytoskeleton-associated polysome fractions; lanes 3, 6, 9, and 12, membrane-associated polysome fractions. Source of polysome population and time after PH are indicated at top and at bottom, respectively. miRNAs identified by Northern blot or the 5S rRNA are indicated at right.
Not all miRNAs exhibited a dramatic increase of their relative abundance in the cytoskeleton- or membrane-associated polysome population after PH. For example, miR-451 levels were very low in the cytoskeleton- and membrane-associated polysome populations at time 0, and changes in distribution during liver regeneration were minor (Table 4; Fig. 8). miR-451 Northern blotting results were consistent with the microarray data (Fig. 9C). Together, these data indicate that the individual polysome populations exhibit discrete miRNA profiles, which undergo significant changes during rat liver regeneration after PH.
DISCUSSION
Liver regeneration after 70% PH provides an opportunity to study modulation of gene expression in a semisynchronous cell population in which ∼95% of the hepatocytes move from a quiescent to a replicative state. It is well established that posttranscriptional regulation of gene expression plays a significant role in this process. During liver regeneration, c-myc and p53 mRNA and protein levels are markedly uncoupled (10, 33). Thus it was important to determine whether this uncoupling is related to the association of transcript with specific polysome populations. In this study, we fractionated polysomes into free and cytoskeleton- and membrane-bound populations in quiescent and regenerating rat liver after 70% PH and characterized their RNA content. In quiescent liver, the total polysome RNA was differentially distributed in the three populations and during the first 24 h after PH. We then identified both c-myc and p53 transcripts in the discrete polysomes and established that their abundance in each population was modulated after PH. Unique patterns of significantly increased or decreased levels for each transcript was determined relative to quiescent liver at 3, 6, and 24 h after PH. The dramatic changes observed for p53 and to a lesser extent c-myc mRNAs in the membrane-bound populations were the most disparate from the total RNA associated with each population. These differences in expression patterns suggest that the RNA flux between the discrete populations is transcript specific rather than global.
In contrast to resting rat liver, ∼70% of c-myc mRNA was associated with the cytoskeletal fraction in cultured cells (19). Moreover, during liver regeneration after PH the increases in transcript abundance for c-myc were most pronounced in the free and membrane-bound populations. No reports have characterized the association of p53 transcript with the different polysome populations either in cultured cells or in vivo. Similar to c-myc, p53 mRNA was associated with all three populations, yet its abundance was significantly greater in the free fraction. A substantial modulation of p53 mRNA was observed in the free and membrane polysomal fractions, while the cytoskeletal fraction remained at or below basal levels. The peak polysomal abundance of c-myc transcript levels occurred at the same time as total cellular levels after PH yet were 17-fold increased over basal level versus a 50-fold increase for total cellular levels (26). Interestingly, the largest increase in polysome-associated p53 mRNA was at 3 h after PH, yet total cellular level peaks at 6 and 24 h were ∼2.5-fold elevated over those at 3 h. This suggests that polysomal levels for a specific transcript do not necessarily mirror total cellular abundance for peak expression after PH. Moreover, changes in transcript abundance for both p53 and c-myc were particularly reflected in the free and membrane-associated populations in this model of cell replication.
Our results showed little correlation between peak protein and transcript levels in a discrete polysome population. Substantial changes in the ratio of transcript and protein were observed in all three populations after PH, with the largest discrepancies noted in the cytoskeleton-bound and free populations. This was particularly striking as the levels of protein associated with the three fractions in quiescent liver reflected transcript abundance in the same population. Both c-myc and p53 transcript and protein association with the free, cytoskeletal, or membrane-bound polysomes was lost when the polysomes were disrupted by EDTA treatment before block gradient purification. Interestingly, the percentage of ribosomes present per microgram of total polysomal protein was increased in the cytoskeletal relative to the free fraction, and the membrane-bound relative to the cytoskeleton-bound fraction, inverse to the total RNA concentration in the different populations. Thus the polysome population with which a specific mRNA is associated appears to significantly impact protein expression and may, in part, be responsible for observed uncoupling of protein and transcript levels for c-myc and p-53 in the regenerating liver.
A proposed function for the polysomal sorting of transcripts is the compartmentalization of protein synthesis to prevent disregulation of cellular function (17, 19). To achieve this end, signals present in the 3′-UTR are essential in directing the intracellular localization of transcript and providing a method for strict translational control. This, coupled with the modulation of total and polysome-associated RNA for c-myc and p-53 (26) in protein expression within the same fraction during liver regeneration, suggested a reversible mechanism of translational repression. Both p53 and c-myc are subject to translational regulation that is uniquely specific to their 3′-UTRs (8, 12, 13, 41). Although translational control of p53 and c-myc is known to occur via the RNA binding proteins HuR and AUF1/TIAR, respectively (14, 30), neither HuR nor AUF1 expression is increased during liver regeneration after PH (27). In addition, we looked at the phosphorylation status of the polysome-associated eIF2-α (6) using phosphoSer51-specific antibodies and found no significant modulation of levels after PH in the different polysome fractions (data not shown). This suggested that alternate forms of translational repression might be occurring.
On the basis of a recent study describing the polysomal regulation of miRNA activity in human cells (31), we characterized the miRNA populations associated with the three discrete polysome fractions in quiescent and regenerating liver. Our results showed that global miRNA distribution to the free and cytoskeleton- and membrane-bound polysomes changed dramatically after PH. The free polysomes maintained the highest concentrations of miRNAs, but relative to free, the membrane- and to a lesser extent cytoskeleton-bound polysomes showed increased levels of miRNAs. As listed in Table 2, more than half of the miRNAs that gave clear hybridization signals in our microarrays enhanced their relative abundance in membrane- and/or cytoskeleton-bound polysomes. PH did not significantly alter the aggregate miRNA levels in the three polysome populations or in total liver lysates (data not shown), but the relative miRNA abundance in the different populations was already changed by 3 h. Such changes were confirmed by Northern blot analyses of selected miRNAs, indicating the validity of the array analyses.
Movement of some miRNAs from the free polysomes to the cytoskeleton- and membrane-bound populations probably accounts in part for the changes in relative miRNA abundance in different populations after PH. Non-polysome-associated miRNAs may be preferentially recruited to the cytoskeleton- and membrane-bound polysome populations, or miRNAs associated with certain polysome population(s) may be retained. It remains to be determined which of the miRNAs specifically target rat c-myc or p53 (20) as a potential mechanism for uncoupling of transcript and protein expression in the regenerating liver. However, let-7a is known to target and modulate c-myc expression in Burkitt lymphoma (34) as well as colon cancer (1) cells. Moreover, let-7a has also been shown to directly modulate translational repression at the polysomal level (5). Recently, miR-125b overexpression was shown to repress p53 translational activity in human cells and p53 protein levels (28). Together, these findings suggest that translational modulation of c-myc and p53 mRNAs observed during liver regeneration after PH could, in part, be controlled by let-7a and miR-125b, respectively. This notion is supported by the concurrent increase in let-7a miRNA in the cytoskeleton- and membrane-bound populations and miR-125b in the membrane-bound fractions associated with decreased expression of the proteins even when transcript levels for c-myc and p53 were increased.
In conclusion, the regenerating rat liver after PH provides an excellent in vivo model to investigate the myriad facets that control posttranscriptional gene expression (24, 29). It is readily apparent that the dynamic flux between the different polysome populations may be a key factor in that process. Polysomal association appears to significantly modulate the translational activity of c-myc and p53 and likely other mRNAs. Similarly, the intracellular trafficking of miRNAs between the different populations is almost invariably involved in the translational activity of numerous transcripts. One obvious possibility for the polysomal flux of miRNAs is to simply follow the movement of their target mRNAs. The increased presence of mRNAs could draw their complementary miRNAs to the corresponding locations to specifically dampen their translational activity. This provides not only a rapid response but also a reversible method of translational repression (21). The regulation of posttranscriptional gene expression is certainly complex, and now involves the interplay between polysomes and miRNAs. Moreover, a recent study using a conditional Dicer knockout mouse indicates that miRNAs play essential roles in hepatocyte survival, metabolism, tumor suppression, and developmental gene regulation (36), leaving little doubt they play a key role in regulating liver regeneration. Further studies will define not only the role of miRNAs in regulating c-myc and p53 but also the significant uncoupling of transcript and protein expression for the myriad other genes in the regenerating rat liver.
GRANTS
This study was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-44649-06 and R01-DK-081865-01 and Medica Foundation Grant 2006-100412 (to C. J. Steer) and by National Institute of Drug Abuse Center Grant DA-011806 and US Department of Defense Army Grant W81XWH-07-1-0183 (to Y. Zeng).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Akao Y, Nakagawa Y, Naoe T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull 29: 903–906, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Brewer G, Ross J. Messenger RNA turnover in cell-free extracts. Methods Enzymol 181: 202–209, 1990 [DOI] [PubMed] [Google Scholar]
- 3.Chabanon H, Mickleburgh I, Hesketh J. Zipcodes and postage stamps: mRNA localisation signals and their trans-acting binding proteins. Brief Funct Genomic Proteomic 3: 240–256, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Sparks JD, Yao Z, Fisher EA. Hepatic polysomes that contain apoprotein B mRNA have unusual physical properties. J Biol Chem 268: 21007–21013, 1993 [PubMed] [Google Scholar]
- 5.Chu CY, Rana TM. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol 4: e210, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clemens MJ. Initiation factor eIF2-α phosphorylation in stress responses and apoptosis. Prog Mol Subcell Biol 27: 57–89, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Corral-Debrinski M. mRNA specific subcellular localization represents a crucial step for fine-tuning of gene expression in mammalian cells. Biochim Biophys Acta 1773: 473–475, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Dalgleish GD, Veyrune JL, Accornero N, Blanchard JM, Hesketh JE. Localisation of a reporter transcript by the c-myc 3′-UTR is linked to translation. Nucleic Acids Res 27: 4363–4368, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desjardins P, Savoie C, Doucet J, Gauthier D. Isolation and characterization of hamster brain polyribosome-cytomatrix complexes. Neurochem Int 21: 21–27, 1992 [DOI] [PubMed] [Google Scholar]
- 10.Fan G, Xu R, Wessendorf MW, Ma X, Kren BT, Steer CJ. Modulation of retinoblastoma and retinoblastoma-related proteins in regenerating rat liver and primary hepatocytes. Cell Growth Differ 6: 1463–1476, 1995 [PubMed] [Google Scholar]
- 11.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology 43: S45–S53, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fu L, Benchimol S. Participation of the human p53 3′UTR in translational repression and activation following γ-irradiation. EMBO J 16: 4117–4125, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu L, Minden MD, Benchimol S. Translational regulation of human p53 gene expression. EMBO J 15: 4392–4401, 1996 [PMC free article] [PubMed] [Google Scholar]
- 14.Galban S, Martindale JL, Mazan-Mamczarz K, Lopez de Silanes I, Fan J, Wang W, Decker J, Gorospe M. Influence of the RNA-binding protein HuR in pVHL-regulated p53 expression in renal carcinoma cells. Mol Cell Biol 23: 7083–7095, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb E. The 3′ untranslated region of localized maternal messages contains a conserved motif involved in mRNA localization. Proc Natl Acad Sci USA 89: 7164–7168, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res 22: 842–849, 1962 [PubMed] [Google Scholar]
- 17.Hesketh J. 3′-Untranslated regions are important in mRNA localization and translation: lessons from selenium and metallothionein. Biochem Soc Trans 32: 990–993, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol 12: 186–202, 1931 [Google Scholar]
- 19.Hovland R, Hesketh JE, Pryme IF. The compartmentalization of protein synthesis: importance of cytoskeleton and role in mRNA targeting. Int J Biochem Cell Biol 28: 1089–1105, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Hsu PW, Huang HD, Hsu SD, Lin LZ, Tsou AP, Tseng CP, Stadler PF, Washietl S, Hofacker IL. miRNAMap: genomic maps of microRNA genes and their target genes in mammalian genomes. Nucleic Acids Res 34: D135–D139, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Liang Z, Yang B, Tian H, Ma J, Zhang H. Derepression of microRNA-mediated protein translation inhibition by apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G) and its family members. J Biol Chem 282: 33632–33640, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Kalscheuer S, Zhang X, Zeng Y, Upadhyaya P. Differential expression of microRNAs in early-stage neoplastic transformation in the lungs of F344 rats chronically treated with the tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. Carcinogenesis 29: 2394–2399, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kren BT, Kumar NM, Wang SQ, Gilula NB, Steer CJ. Differential regulation of multiple gap junction transcripts and proteins during rat liver regeneration. J Cell Biol 123: 707–718, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kren BT, Rodrigues CM, Setchell KD, Steer CJ. Modulation of steady-state messenger RNA levels in the regenerating rat liver with bile acid feeding. Liver Transpl 7: 321–334, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Kren BT, Steer CJ. Posttranscriptional regulation of gene expression in liver regeneration: role of mRNA stability. FASEB J 10: 559–573, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Kren BT, Trembley JH, Steer CJ. Alterations in mRNA stability during rat liver regeneration. Am J Physiol Gastrointest Liver Physiol 270: G763–G777, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Lafon I, Carballes F, Brewer G, Poiret M, Morello D. Developmental expression of AUF1 and HuR, two c-myc mRNA binding proteins. Oncogene 16: 3413–3421, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, Lodish HF, Lim B. MicroRNA-125b is a novel negative regulator of p53. Genes Dev 23: 862–876, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leeds P, Kren BT, Boylan JM, Betz NA, Steer CJ, Gruppuso PA, Ross J. Developmental regulation of CRD-BP, an RNA-binding protein that stabilizes c-myc mRNA in vitro. Oncogene 14: 1279–1286, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Liao B, Hu Y, Brewer G. Competitive binding of AUF1 and TIAR to MYC mRNA controls its translation. Nat Struct Mol Biol 14: 511–518, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Maroney PA, Yu Y, Fisher J, Nilsen TW. Evidence that microRNAs are associated with translating messenger RNAs in human cells. Nat Struct Mol Biol 13: 1102–1107, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Michalopoulos GK. Liver regeneration. J Cell Physiol 213: 286–300, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morello D, Lavenu A, Babinet C. Differential regulation and expression of jun, c-fos and c-myc proto-oncogenes during mouse liver regeneration and after inhibition of protein synthesis. Oncogene 5: 1511–1519, 1990 [PubMed] [Google Scholar]
- 34.Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res 67: 9762–9770, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Schiavi SC, Belasco JG, Greenberg ME. Regulation of proto-oncogene mRNA stability. Biochim Biophys Acta 1114: 95–106, 1992 [DOI] [PubMed] [Google Scholar]
- 36.Sekine S, Ogawa R, Ito R, Hiraoka N, McManus MT, Kanai Y, Hebrok M. Disruption of Dicer1 induces dysregulated fetal gene expression and promotes hepatocarcinogenesis. Gastroenterology 136: 2304–2315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5: 836–847, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Trembley JH, Ebbert JO, Kren BT, Steer CJ. Differential regulation of cyclin B1 RNA and protein expression during hepatocyte growth in vivo. Cell Growth Differ 7: 903–916, 1996 [PubMed] [Google Scholar]
- 39.Trembley JH, Kren BT, Steer CJ. Posttranscriptional regulation of cyclin B messenger RNA expression in the regenerating rat liver. Cell Growth Differ 5: 99–108, 1994 [PubMed] [Google Scholar]
- 40.Vedeler A, Pryme IF, Hesketh JE. Compartmentalization of polysomes into free, cytoskeletal-bound and membrane-bound populations. Biochem Soc Trans 19: 1108–1111, 1991 [DOI] [PubMed] [Google Scholar]
- 41.Veyrune JL, Hesketh J, Blanchard JM. 3′ Untranslated regions of c-myc and c-fos mRNAs: multifunctional elements regulating mRNA translation, degradation and subcellular localization. Prog Mol Subcell Biol 18: 35–63, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA 100: 9779–9784, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]