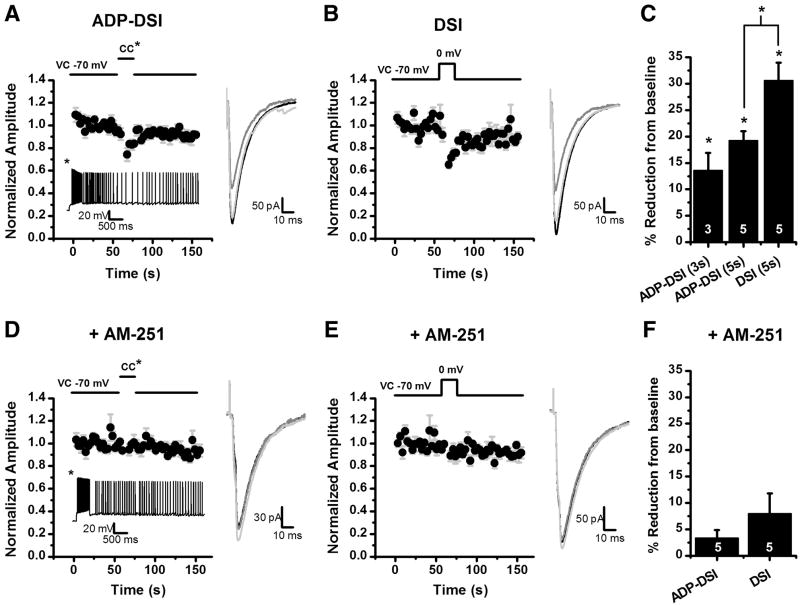
Figure 5. ADP can cause a release of eCBs resulting in DSI.
eIPSCs were evoked with a concentric bipolar stimulator placed in the hilus at 0.33 Hz in the presence of 5 μM muscarine or 3 μM carbachol. A: For ADP-DSI a mixed mode protocol was used. Following a baseline the cell was switched to current clamp and a 500 ms depolarizing pulse was applied evoking a train of action potentials and resulting in an ADP. Five seconds after the depolarization the cell was switched back to voltage clamp and eIPSCs were evoked again at 0.33 Hz. The ADP resulted in a transient decrease in the eIPSC amplitude (DSI). The inset below the graph is an ADP from a representative cell in current clamp. B: For the DSI protocol, instead of switching to current clamp the cell was depolarized in voltage clamp from −70 mV to 0 mV for 5 seconds resulting in a transient decrease in the eIPSC amplitude (DSI). A–B: The insets to the right of the graphs are the average of 8 sweeps for the baseline (black), 2 sweeps for DSI (dark gray), and 8 sweeps for recovery (light gray). C: Summary plot of the amount of DSI for 3 second and 5 second ADPs and conventional DSI (5 second depolarization). *p<0.05. D: ADP-DSI was attempted after a 20 minute preincubation in 5 μM AM-251, a CB1 receptor antagonist. Induction of a similar ADP no longer resulted in a transient decrease in eIPSCs. Inset below the graph is an ADP from a representative cell in current clamp. E: A depolarization from −70 to 0 mV for 5 seconds also did not result in a transient decrease in eIPSCs in the presence of AM-251. D–E: The insets to the right of the graphs are the average of 8 sweeps for the baseline (black), 2 sweeps for DSI (dark gray), and 8 sweeps for recovery (light gray). F: Summary plot of the amount of DSI under each induction protocol in the presence of AM-251.