Table 1.
Intermolecular Michael additions of carbon nucleophiles to nitrosoalkenes
| entry | ester derivative | nitrosoalkene precursor | product | yield |
|---|---|---|---|---|
| a | 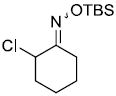 |
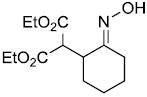 |
95%a | |
| b | 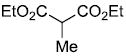 |
 |
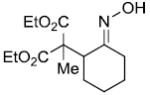 |
84% |
| c | 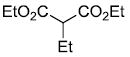 |
 |
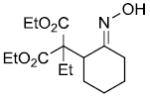 |
69% |
| d |  |
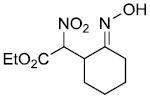 |
57%b,c | |
| e | 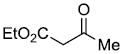 |
 |
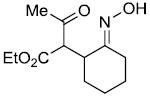 |
71%c |
| f |  |
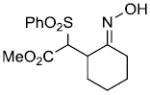 |
95%c | |
| g |  |
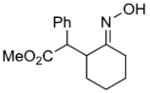 |
79%c | |
| h | 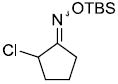 |
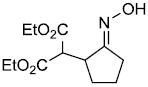 |
85%d,e | |
| i |  |
 |
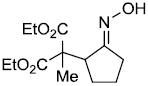 |
72% |
| j |  |
 |
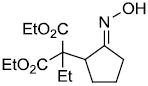 |
73% |
| k |  |
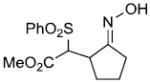 |
82%c | |
| l |  |
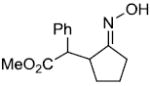 |
55%c,d | |
| m | 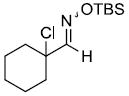 |
 |
64%d,e | |
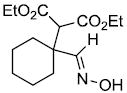
| n |  |
 |
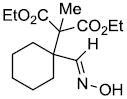 |
74% |
| o |  |
 |
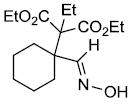 |
69%d |
| p |  |
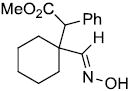 |
63% | |
| q |  |
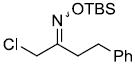 |
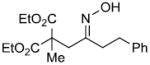 |
88% |
| r |  |
 |
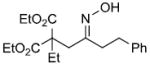 |
71% |
| s |  |
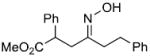 |
75% | |
| t | 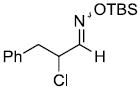 |
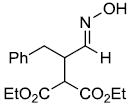 |
51%f | |
| u |  |
 |
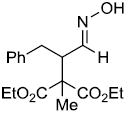 |
69% |
| v |  |
 |
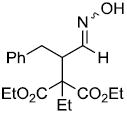 |
68%d,g |
| w |  |
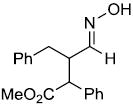 |
75%c,d | |
| x | 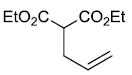 |
 |
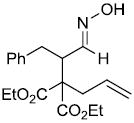 |
67%d |
Use of LiHMDS and NaHMDS gave yields of 91% and 94%, respectively.
No desired product was formed when using LiHMDS or NaHMDS.
An accurate stereochemical assignment could not be made since the products exist as a complex mixture of E/Z-isomers and/or diastereomers which were not separable by column chromatography.
2 eq of KHMDS and 2 eq of ester derivative were used.
The deprotonation step was performed at 0 °C to prevent freezing of the reaction mixture.
Use of LiHMDS and NaHMDS gave 34% and 51%, respectively.
E:Z ratio could not be determined.
