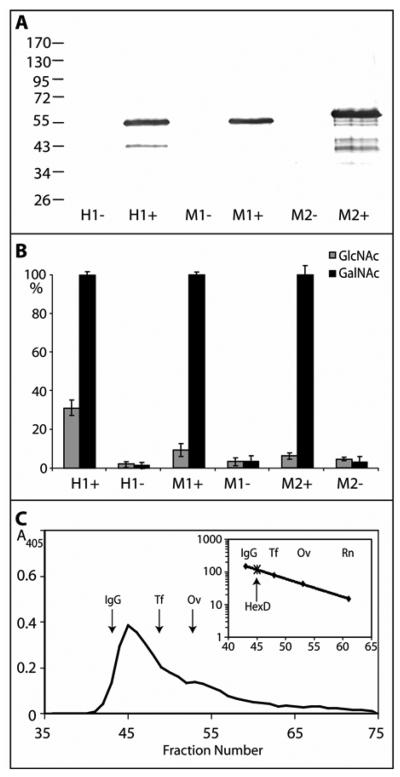
Figure 2. Recombinant expression of mammalian hexosaminidase D in E. coli.
Two clones encoding His-tagged forms of either the short (M1; 486 residues) or long (M2; 559 residues) forms of murine hexosaminidase D and of one clone encoding human hexosaminidase D (H1; 486 residues) were used to transform E. coli and lysates were prepared after cultivation for three hours in the presence (+) or absence (−) of IPTG. (A) Western blotting of transformed E. coli indicates expression of proteins of the expected sizes; (B) assay with either p-nitrophenyl-β-N-acetylgalactosaminide or p-nitrophenyl-β-N-acetylglucosaminide for one hour at 37 °C shows induction of especially p-nitrophenyl-β-N-acetylgalactosaminidase activity with mammalian hexosaminidase D clones. Duplicate data were normalised with 100% corresponding to the activity of the induced cell lysate towards p-nitrophenyl-β-N-acetylgalactosaminide in that experiment and the averaged normalised data are from two independent experiments; therefore, each point corresponds to four single enzymatic assays. (C) Gel filtration analysis of the short form of the murine hexosaminidase D (M1) on a Sephacryl S-200 Superfine column calibrated (see insert with scale in terms of Mr × 10−3) with IgG (IgG; 150 kDa), apo-transferrin (Tf; 80 kDa), ovalbumin (Ov; 43 kDa), and ribonuclease B (Rn; 15 kDa). Hexosaminidase activity was recorded at 405 nm; the presence of the protein with the expected molecular mass was verified by Western blotting (data not shown).