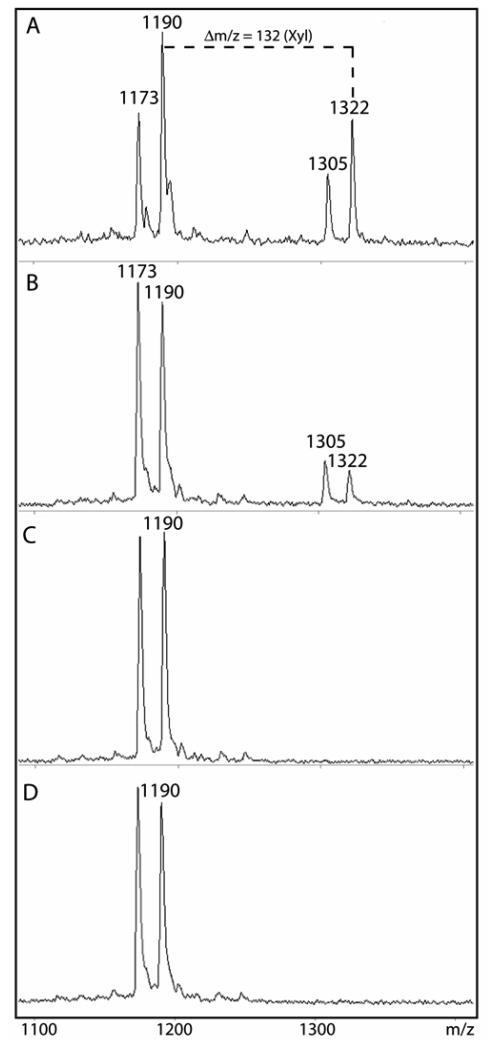
Figure 4. Assay of recombinant human xylosyltransferase II expressed in mammalian and yeast systems using the bikunin peptide.
Xylosyltransferase activity of concentrated supernatants of either (A) pgsA-745 or (B) Pichia transformed with vectors encoding XT-II was measured overnight at 37 °C using the bikunin peptide substrate in the presence of UDP-Xyl, HEPES buffer, pH 8, and Mn(II). Controls with either (C) Pichia-expressed XT-II incubated in the absence of UDP-Xyl or of (D) Pichia transformed with empty vector were also performed. The primary xylosylation product has an m/z of 1322. In all cases, a secondary reaction resulting in a shift in m/z of −17 (species of 1173 and 1305) was also, as previously described (Ref. 19), observed and is presumed to be due to the conversion of the N-terminal glutamine residue of both the acceptor and product to pyroglutamate; this modification has no obvious effect on the xylosyltransferase reaction.