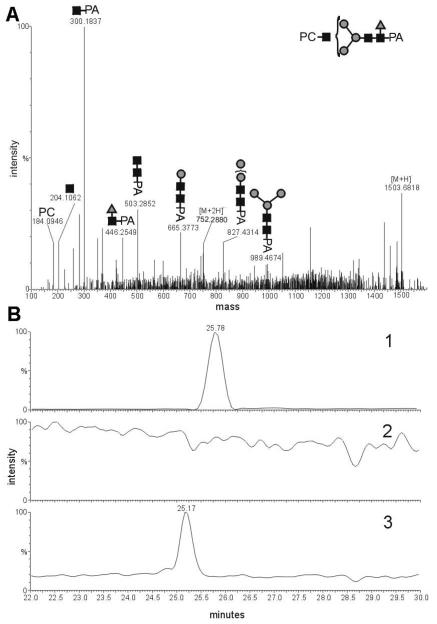
Figure 5. Analysis of an Ascaris suum N-glycan modified by phosphorylcholine and fucose.
A) CID-MS-MS analysis of the presumed HexNAc3Hex3Fuc1PC1-PA in its [M+2H]2+ form (m/z 752.2880); B) LC-ESI-MS ion trace of 2-aminopyridine labeled Ascaris suum N-glycans. Chromatogram 1 shows the trace of m/z 752.30 (HexNAc3Hex3Fuc1PC1) of a 2-aminopyridine N-glycan fraction, purified by the “two-dimensional” mapping technique, before treatment with α-fucosidase. Chromatogram 2 shows the trace m/z 752.30 after incubation with α-fucosidase, showing that structures with this m/z were completely digested by this treatment. Chromatogram 3 shows the ion trace of m/z 679.27 (HexNAc3Hex3PC1) of the same fraction as in chromatogram 1, but after treatment with α-fucosidase and indicates a shift to lower retention time.