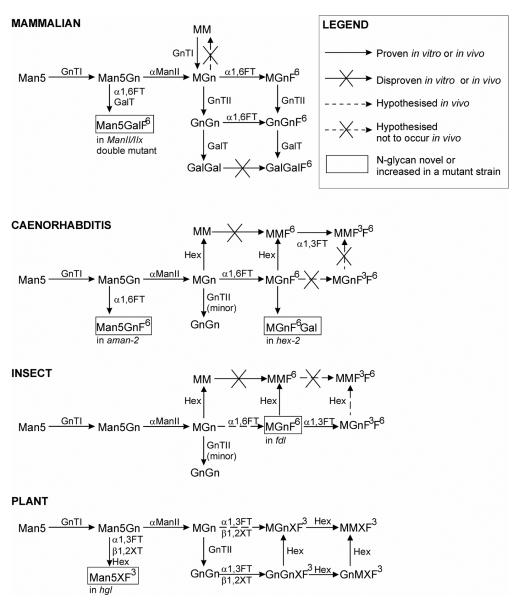
Scheme II. Putative pathways towards paucimannosidic glycans in nematodes, plants and insects.
Only the major pathways are shown for mammals (which lack paucimannosidic glycans, but have especially biantennary complex glycans), nematodes, insects and plants, as well as pathways which are revealed in strains lacking either Golgi mannosidase II from mammals (mannosidase II/IIx double mutant emybros), nematodes (aman-2) and plants (hgl) and hexosaminidase mutants from nematodes (hex-2; this study) and insects (fdl). Whereas both fucosylated and non-fucosylated paucimannosidic structures naturally dominate in nematodes and insects (especially MM and MMF6), truncated N-glycans in plants carry xylose and fucose residues; the hexosaminidases from nematodes and insects only remove the GlcNAc attached to the α1,3-antenna, whereas those from plants display no distinct arm preference. On the other hand, core α1,6-fucosyltransferases from nematodes and insects, core α1,3-fucosyltransferases from plants and insects and β1,2-xylosyltransferases from plants cannot transfer to MM (in some cases, transfer to MGn or Man5Gn has been proven in vitro), whereas the nematode core α1,3-fucosyltransferase can transfer, at least in vitro, to MM (but not MGn). The action of the hexosaminidases in nematodes, plants and insects is therefore necessary to explain the range of N-glycan structures observed. No glycans from an insect Golgi mannosidase II mutant or a plant hexosaminidase mutant have yet been analysed; thus, the in vivo affect of such defects is still unknown.