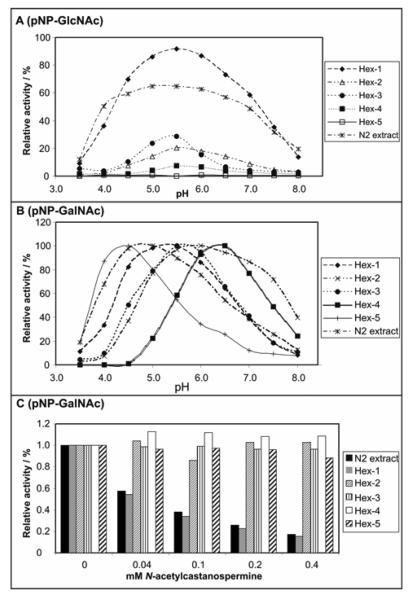
Figure 2. Enzymatic properties of Caenorhabditis class 20 hexosaminidases.
(A) pH dependency of activity towards p-nitrophenyl-β-N-acetylglucosaminide (pNP-GlcNAc) of native and recombinant C. elegans hexosaminidases assayed at 37 °C over a one-hour period using a range of McIlvaine buffers; (B) pH dependency of activity towards p-nitrophenyl-β-N-acetylgalactosaminide (pNP-GalNAc) of native and recombinant C. elegans hexosaminidases assayed at 37 °C over a one-hour period using a range of McIlvaine buffers; (C) sensitivity of native and recombinant C. elegans hexosaminidases assayed at 37 °C over a one-hour period at optimal pH using p-nitrophenyl-β-N-acetylgalactosaminide as substrate and various concentrations of N-acetylcastanospermine as potential inhibitor. Crude culture supernatants of yeast transformed with hex partial open reading frames (1:10 diluted in the case of hex-1 and hex-2) or an extract of wild-type N2 worms were used as the enzyme sources; in all cases, data was recalculated with the condition yielding the highest absorbance at 405 nm with p-nitrophenyl-β-N-acetylgalactosaminide as substrate being normalised to 100%.