Abstract
Recent studies suggest that hyperinsulinemia and insulin resistance are linked to Alzheimer’s disease (AD). In this study, we used Tg2576 transgenic (Tg) mice, a widely used transgenic mouse model for AD, to explore the relationship between increased amyloid β-peptide (Aβ) and insulin resistance. When fed a high-fat diet (HFD), Tg mice developed obesity and insulin resistance at 16 wk of age. Furthermore, HFD-fed Tg mice displayed abnormal feeding behavior and increased caloric intake with time. Although caloric intake of HFD-fed Tg mice was similar to that of normal diet-fed Tg or wild-type mice during 4 to 8 wk of age, it increased sharply at 12 wk, and went up further at 16 wk, which paralleled changes in the level of Aβ40 and Aβ42 in the brain of these mice. Limiting food intake in HFD-fed Tg mice by pair-feeding a caloric intake identical with that of normal diet-fed mice completely prevented the obesity and insulin intolerance of HFD-fed Tg mice. The hypothalamus of HFD-fed Tg mice had a significant decrease in the expression of the anorexigenic neuropeptide, brain-derived neurotrophic factor, at both the mRNA and protein levels. These findings suggest that the increased Aβ in the brain of HFD-fed Tg2576 mice is associated with reduced brain-derived neurotrophic factor expression, which led to abnormal feeding behavior and increased food intake, resulting in obesity and insulin resistance in these animals.
High fat diet feeding leads to obesity and insulin resistance in the Tg2576 Alzheimer’s disease mouse model by suppressing hypothalamic brain-derived neurotrophic factor expression and upregulating their food consumption.
Hyperinsulinemia and insulin resistance are key pathophysiological factors associated with type 2 diabetes mellitus. Recent studies suggest that these metabolic abnormalities are also linked to Alzheimer’s disease (AD). Two population-based cohort studies demonstrated an increased risk of dementia among patients with type 2 diabetes, independent of the risk for vascular dementia (1,2). What further piques our interest in the role of insulin resistance in AD is the fact that the gene that encodes the insulin-degrading enzyme (IDE) lies in a region of chromosome 10q, which is linked to late-onset AD (3). IDE is a zinc-metalloprotease that degrades insulin as well as small peptides, which have the capacity to form β-pleated sheet structure, including the amyloid β-peptide (Aβ), which forms the characteristic plaques of AD brain. It has been hypothesized that binding of insulin to IDE reduces Aβ degradation under conditions of hyperinsulinemia and promotes amyloid plaque formation (4,5). Hyperinsulinemia has been shown to be a risk factor for AD among nondiabetics, especially individuals lacking the ApoE-ε4 allele (6,7). Therefore current evidence supports a relationship between insulin resistance, altered brain IDE levels, and clearance of Aβ in AD brain. Furthermore, type 2 diabetes appears to be a significant risk factor for clinical AD, although the mechanism through which diabetes impacts AD is poorly understood.
Insulin resistance has been reported to modify the production and degradation of Aβ in brain using the transgenic (Tg) Tg2576 mouse model of AD (8,9,10). The Tg2576 mice are one of the most widely used Tg mouse models for AD (11). They show progressive declines in memory function after 6 month of age, at a time when Aβ levels begin to rise rapidly (12). In this model, diet-induced insulin resistance was found to be associated with reduced neuronal insulin receptor signaling, impaired performance in a spatial water maze, and increased Aβ levels in the brain resulting from enhanced γ-secretase activity and reduced IDE activity (8). Also, connective tissue growth factor expression was found to correlate with the AD dementia and amyloid plaque neuropathology (10). Pedersen et al. showed that Tg2576 mice exhibit aberrant stress response and peripheral insulin resistance that is correlated with abnormally high glucocorticoid levels during stress in these mice (9). It is unclear whether either condensation of Aβ or amyloid plaque in the brain affects peripheral insulin sensitivity.
In this study, we used AD model Tg2576 mice to explore the relationship between increased Aβ and insulin resistance. The findings presented are the first to indicate a strong relationship between increased food intake and Aβ condensation in the brain, linking Aβ with increased food intake and insulin resistance, which may in turn boost Aβ formation in the brain.
Materials and Methods
Animals and dietary conditions
Tg2576 mice, a Tg line that expresses human amyloid precursor protein with a familial APP mutation (K670N, M671L) and nontransgenic colony control (wild-type, WT) were purchased from Taconic Farms (Germantown, NY). Tg2576 male mice were more aggressive than female mice and were housed individually to avoid fighting. Because individual housing could be stressful to them, we used female mice for our experiment and kept them in the same cage. Four-wk-old female mice were exposed to either a high fat diet (HFD) that had 42% of calories from fat (Harlan Laboratories, Inc., Indianapolis, IN; product no. TD88137) or a standard rodent laboratory diet that had 15% of calories from fat (Harlan Laboratories, Inc.; product no. 2020X) and then assessed at 16 wk of age. For pair-feeding (PF) experiment, we measured caloric intake every 2 wk, and down-adjusted the total amount of feed in the HFD to have an identical caloric content as that of normal diet (ND) fed to Tg2576 and WT mice. Body weights were monitored every 4 wk. Echo Magnetic Resonance Imaging (Echo Medical System, Houston, TX) was used for body composition analysis. Mice were anesthetized with 2,2,2-Tribromoethanol (Sigma, St. Louis, MO) and killed by decapitation at the indicated times. Tissues were fixed in 4% paraformaldehyde or frozen in liquid nitrogen and stored for further analysis. Whole hypothalami were dissected from the brain and used for Western blot. All experimental procedures were carried out under a protocol approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine and were in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Antibodies and assays
Anti-brain-derived neurotrophic factor (BDNF) polyclonal antibodies were purchased from Chemicon (Temecula, CA). Serum samples were collected after a 6-h fasting. Serum triglycerides (Infinity assay by Thermo Electron, Melbourne, Australia), nonesterified free-fatty acids (Wako Chemicals USA, Hercules, CA), and glycerol (Sigma) were measured by colorimetric assay. For serum corticosterone measurements, blood was collected at 0700 h, 1900 h in the fed state, as well as at 0700 h after an overnight fast. Serum insulin (Mercodia, Uppsala, Sweden), corticosterone (Oxford Biomedical Research, Oxford, MI), and leptin (R and D Systems, Inc., Minneapolis, MN) were measured using an enzyme immunoassay kit according to the manufacturer’s instructions. For ghrelin determination, we collected blood in EDTA-containing tubes at 0700 h in the fed state and after a 24-h fast. Samples were centrifuged, and aliquoted into polypropylene vials and stored at 4 C for further analysis. Total serum ghrelin levels were measured by EIA using kits purchased from Linco Research (St. Charles, MO).
Glucose and insulin tolerance test
Glucose tolerance test (GTT) was performed by ip injection of 1.0 g/mouse kg d-glucose after a 6-h fast. Insulin tolerance test (ITT) was performed after a 6-h fast by injecting the mice with 0.5 U/mouse kg humulin R insulin ip (Eli Lilly and Co., Indianapolis, IN) as described previously (13).
Food intake
Mice were equilibrated in separate housing for 2 d and then the weights of food were measured every 24 h for 7 d or 3 h for 3 d. To calculate caloric intake, HFD had 4.5 kcal/g, whereas ND had 3.3 kcal/g of physiologic fuel value.
Behavioral satiety sequence (BSS)
Mice were habituated to a 40-min daily presentation of wet mash feed (1 part of powdered laboratory diet mixed with 2.4 parts tap water by weight) for a week. After 60-min fasting, a dish containing a preweighed amount of wet mash was presented. The animals were observed for a 40-min period to record a BSS and scored for each of four behaviors (feed, active, groom, and inactive). After the test session, wet mash was removed and reweighed as described previously (14).
Mouse activity
Activity was determined for singly housed mice using VersaMax equipment and software (AccuScan Instruments, Columbus, OH). Data were collected over 22 h from 1500 h to 1300 h the next day. The dark cycle of the room was from 1900 h to 0700 h.
Quantitative RT-PCR (qPCR)
Total RNA was extracted from hypothalamus using Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA) as per the manufacture’s instructions. RNA was treated with DNase I and reverse transcribed using Superscript III First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). qPCR was performed using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and Mx3000P quantitative PCR machine (Stratagene). GAPDH, hmbs, and eef1g were used as the housekeeping genes.
Quantification of Aβ
The brain was homogenized in six volumes of 2% sodium dodecyl sulfate with protease inhibitor and centrifuged 100,000 × g at 4 C for 1 h. The supernatant was then collected and diluted 50 times in 0.02 m sodium phosphate (pH 7.0), 0.4 m NaCl, 2 mm EDTA, 0.2% BSA, 0.05% CHAPS, 0.05% sodium azide and 0.05% sodium dodecyl sulfate containing 0.05% sodium dodecyl sulfate. The pellet was sonicated in 70% formic acid and centrifuged 100,000 × g at 4 C for 1 h. The supernatant was then collected, neutralized by 1:20 dilution into 1 m Tris (pH 11.0), and then diluted 2.5 times in 0.02 m sodium phosphate (pH 7.0), 0.4 m NaCl, 2 mm EDTA, 0.2% BSA, 0.05% CHAPS, 0.05% sodium azide, and 0.05% sodium dodecyl sulfate. Total Aβ40 or Aβ42 content in brain lysates was measured by sandwich ELISA (BioSource, Camarillo, CA) according to the manufacturer’s instructions. Using the wet weight of brain in the original homogenate, the final values of Aβ in brain were expressed as picogram per gram wet weight. Nontransgenic tissues were processed identically in parallel with the Tg tissues.
Immunofluorescence of brain tissue
Freshly harvested mouse brain hemispheres are immersion-fixed overnight in 4% paraformaldehyde and then sectioned in the colonal plane. Mounted tissue sections were permeabilized in PBS [137 mm NaCl, 2.7 mm KCl, 8.1 mm Na2HPO4, and 1.5 mm KH2PO4 (pH 7.4)] containing 0.1% Triton X-100, and washed three times with PBS, followed by blocking with PBS containing 10% fetal bovine serum for 60 min. The sections were initially incubated overnight with the anti-BDNF rabbit antibodies, and Alexa Fluor 594-labeled goat antirabbit IgG antibody (Molecular Probes, Eugene, OR) was used as secondary antibodies.
Statistical analysis
All values are expressed as means ± sd with the exception of quantitative PCR results, which are means ± sem. Difference between means was analyzed by using either repeated measure ANOVA, two-way ANOVA, post hoc tests, or Student’s t test. A different was considered as significant at P < 0.05.
Results
HFD feeding induces excess total body weight and fat mass and hyperinsulinemia in Tg2576 mice
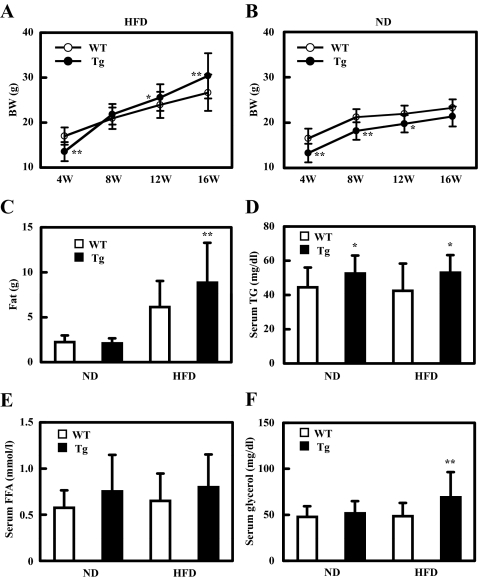
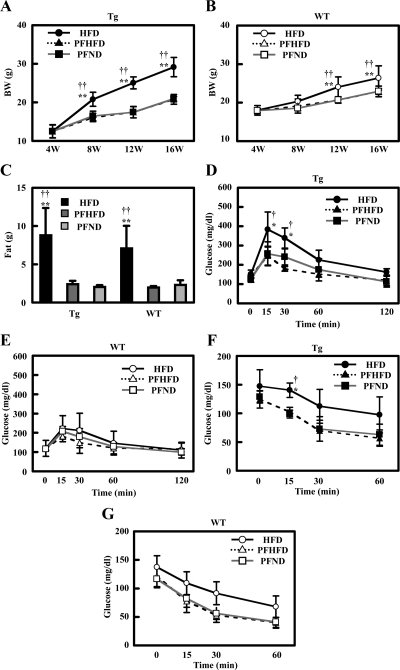
We fed female WT and female Tg2576 mice a 42% fat diet (HFD) at the age of 4 wk. Compared with WT or Tg mice on ND (regular chow), both groups gained weight rapidly in response to the HFD. At the beginning of the HFD, Tg mice weighed significantly less than WT. Their weight surpassed that of 8-wk-old WT by the time they reached 12 wk of age, and the rate of weight gain continued to go up in the next 4 wk (Fig. 1A). In contrast, Tg mice remained consistently lighter than WT when they were maintained on a ND for 12 wk (Fig. 1B). The HDF-induced excess weight gain in Tg mice could be accounted for entirely by increased total body fat as quantified by magnetic resonance imaging at 16 wk of age (Fig. 1C). We also found a similar obese and insulin-resistant phenotype of male Tg mice compared with male WT mice (data not shown). Tg male mice were more aggressive than female mice and needed to be housed individually to avoid fighting. Because individual housing is stressful to the mice, we decided to use only female mice for our experiments and group cage them.
Figure 1.
HFD feeding induces excess total body and fat mass in Tg2576 mice. A and B, Body weight of mice fed with HFD (A) and ND (B). Body weights were monitored every 4 wk (n = 14–27 of each group). C, Weight of fat mass at 16 wk of age (n = 14–27). D, Serum triglyceride at 16 wk of age (n = 17–20 of each group). E, Serum-free fatty acid at 16 wk of age (n = 17–20 of each group). F, Serum glycerol at 16 wk of age (n = 17–20 of each group). In all panels, data are expressed as means ± sd. *, P < 0.05 and **, P < 0.01 vs. WT group.
Serum triglyceride was significantly higher in Tg mice compared with WT mice, whether the mice were on ND or HFD (Fig. 1D). The average serum-free fatty acid level tended to be higher in Tg than in WT mice whether on ND or HFD, but the difference did not reach statistical significance (Fig. 1E). In contrast, serum glycerol level was elevated in HFD-fed Tg mice, but not in ND-fed WT or Tg, or in HFD-fed WT animals (Fig. 1F).
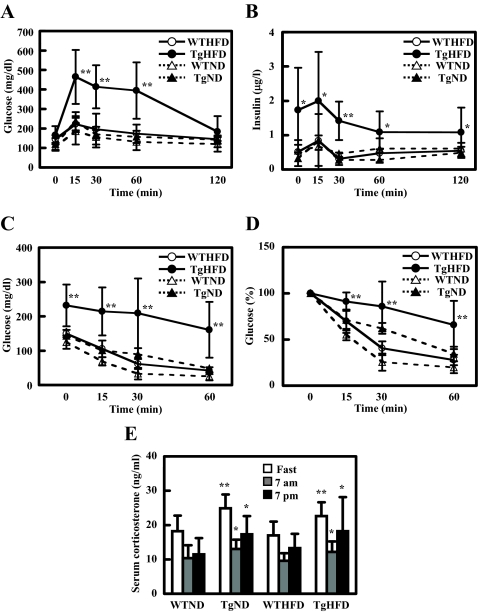
An ip GTT revealed that WT mice on ND or HFD had a normal basal glucose level and glucose response. While on HFD, basal glucose level of Tg mice was higher than that in WT and ND groups; HFD-Tg mice also displayed marked glucose intolerance with elevated blood glucose at 15, 30, and 60 min after glucose load, whereas ND-Tg mice showed a normal glucose response (Fig. 2A). Furthermore, plasma insulin level before and during GTT was significantly higher in HFD-Tg mice when compared with the other groups (Fig. 2B). Homeostatsis model assessment of insulin resistance of HFD-Tg mice tended to be higher (HFD-Tg, 19.1 ± 16.7, HFD-WT, 4.43 ± 2.05, ND-Tg, 2.66 ± 2.48, ND-WT, 3.50 ± 2.42) and QUICKI of HFD-Tg mice were lower than other groups (HFD-Tg, 0.175 ± 0.053, HFD-WT, 0.273 ± 0.078, ND-Tg, 0.478 ± 0.260, ND-WT, 0.313 ± 0.132), but the differences were not significant (P > 0.14, P > 0.17, respectively).
Figure 2.
HFD-feeding induces insulin resistance in Tg2576 mice. A and B, Intraperitoneal glucose tolerance test at 16 wk of age (n = 3–5 of each group). Glucose response is shown in A. Insulin response is shown in B. C and D, Intraperitoneal insulin tolerance test at 16 wk of age (n = 3–5 of each group). Glucose response is shown in C. Change of glucose is shown in D. E, Serum corticosterone at 0700 h with overnight fasting, 0700 h, and 1900 h without fasting at 16 wk of age. In all panels, data are expressed as means ± sd. *, P < 0.05 and **, P < 0.01 vs. WT group.
To determine the role of insulin sensitivity in the glucose response, we performed an ip ITT. At the age of 8 and 12 wk, GTT and ITT were normal among all four groups of mice (data not shown). At 16 wk, HFD-fed Tg mice showed a markedly attenuated glucose lowering after insulin injection. HFD-fed WT and ND-fed Tg mice had slightly higher glucose level than ND-fed WT mice, but there was no significant difference between these groups in the ITT (Fig. 2, C and D). These findings suggest that the impaired glucose tolerance for Tg mice fed with HFD was at least partly the result of insulin resistance.
It was reported that Tg mice showed higher serum corticosterone, which might underlie the insulin resistance of these mice (9). We therefore measured serum corticosterone and found that the level showed diurnal variation, being lower in the morning than in the evening. This variation was modulated by feeding as it was elevated immediately after an overnight fast for all groups (Fig. 2E). Although Tg mice showed significantly higher serum corticosterone level at every time point, there was no difference between HFD- and ND-fed Tg mice (Fig. 2E). Because Tg mice on ND exhibited normal insulin sensitivity, variations in serum corticosterone level likely did not mediate insulin resistance in HFD-fed Tg mice.
Feeding behavior and serum leptin and ghrelin levels in HFD-fed Tg2576 mice
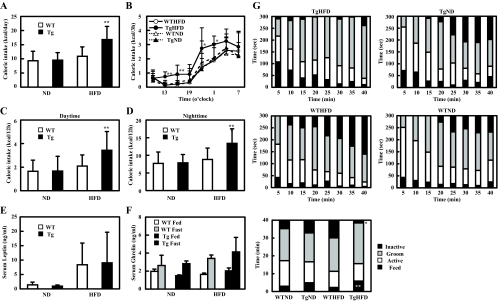
At 16 wk of age, HFD-fed Tg mice had an increased caloric intake compared with the other three groups (ND-fed Tg and WT mice or HFD-WT mice) (Fig. 3A). When we monitored the caloric intake throughout the day, we found that HFD-fed Tg mice consumed more food at all time points compared with other groups. Like the other groups, they displayed diurnal variation in their feeding behavior and consumed more food at night than during the day (Fig. 3, B–D). Caloric intake divided by lean mass weight for HFD-fed Tg mice was also higher than other groups (data not shown). Serum leptin levels were significantly elevated in the HFD groups compared with the ND groups. HFD-fed Tg mice tended to display increased serum leptin levels, but the difference failed to reach statistical significance due to large variations (P > 0.81 respectively) (Fig. 3E). Furthermore, serum ghrelin level was similar between groups (Fig. 3F). These findings suggest that increased caloric intake on HFD-fed Tg mice was not caused by changes in serum leptin or ghrelin levels.
Figure 3.
Food intake and feeding behavior. A, Caloric intake at 16 wk of age. Food intake was measured every day (n = 4–5 of each group). B, Circadian change of caloric intake at 16 wk of age (n = 6–10 of each group). Food intake was measured every 3 h. C and D, Food intake during daytime (C) and nighttime (D). E, Serum leptin at 16 wk of age (n = 17–20 of each group). F, Serum ghrelin at 16 wk of age (n = 4–7 of each group). G, Behavioral satiety sequence at 16 wk of age. Results are shown as the time of behavioral observations in 5-min time bins classified as either inactive, groom, active, or feed (n = 9–12 of each group). Total time of behavioral observations was shown in bottom panel. In all panels, data are expressed as means ± sd. *, P < 0.05 and **, P < 0.01 vs. WT group.
We closely monitored the feeding behavior and analyzed their BSS using the method of Halford et al. (14). There was no difference in the active and grooming time between groups (Fig. 3G). However, HFD-fed Tg mice displayed longer and more sustained feeding action, showing longer feeding time and shorter resting time in comparison with HFD-fed WT mice, whereas ND-fed Tg mice showed slightly longer feeding time compared with ND-WT mice (Fig. 3G). There was no difference in spontaneous activity in the different groups of mice in terms of horizontal activity and distance traveled (data not shown).
Time-dependent change in caloric intake, plasma insulin level, and Aβ content in the brain
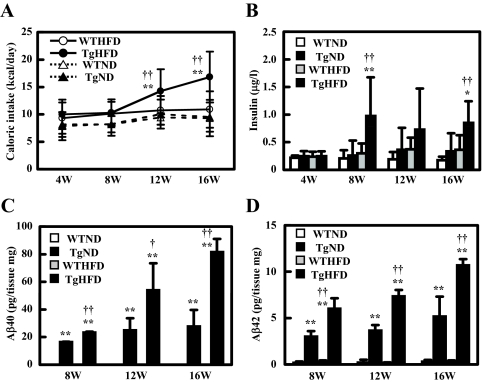
From 4 to 8 wk of age, caloric intake of HFD-fed Tg2576 mice was similar to that of the other groups (Fig. 4A). It increased sharply at 12 wk, and continued to go up further at 16 wk, at which time it was more than 50% higher than that of HFD-fed WT mice (Fig. 4A). We also note that the basal insulin level of HFD-Tg mice was significantly higher than that in the other groups at 8 wk when they all had very similar caloric intake (Fig. 4B). However, as noted previously, GTT and ITT responses were indistinguishable between Tg and WT mice at this time.
Figure 4.
Time course of caloric intake, hyperinsulinemia, and Aβ content in the brain. A, Time course of caloric intake. Food intake was measured every day (n = 4–5 of each group). B, Time course of serum insulin (6 h fasting) (n = 11–12 of each group). C, Time course of Aβ40 extract in brain (n = 3–4 of each group). D, Time course of Aβ42 extract in brain (n = 3–4 of each group). In all panels, data are expressed as means ± sd. *, P < 0.05 and **, P < 0.01 vs. WT group; †, P < 0.05 and ††, P < 0.01 vs. ND group.
IDE has been noted to degrade insulin along with the short peptide Aβ found in excess in the AD brain, and hyperinsulinemia has been postulated to affect Aβ condensation (4,5). Thus, we next examined Aβ content in the brain. We detected essentially no Aβ40 and Aβ42 in WT mice, but easily demonstrated elevated level of Aβ40 and Aβ42 in HFD-fed Tg brain at 8, 12, and 16 wk of age. The Aβ40 and Aβ42 levels of these mice increased markedly at and after 12 wk in HFD-fed Tg mice. As described previously, Aβ in Tg2576 mouse brains were stable before 6 months and increased at 6–7 months (12); also, ND-fed Tg mice showed a much slower increase in the concentration of these peptides (Fig. 4, C and D). These findings suggest that enhanced formation of brain Aβ may be associated with the increased food intake in Tg mice. However, we failed to detect any amyloid plaque in the brain of Tg mice (by thioflavin-S staining) throughout the 16-wk period.
Overeating underlies glucose intolerance and insulin resistance in Tg2576 mice
We performed PF experiments to evaluate the contribution of food intake to obesity and insulin resistance in HFD-fed Tg mice. From 4 wk of age, we measured caloric intake every 2 wk, and provided the same calories of HFD or ND to Tg and WT mice in the subsequent 2 wk. Limiting food intake in Tg mice (PFHFD group) by PF with a caloric intake identical with that of ND-fed mice reduced weight gain to a level identical with that of Tg PFND (Fig. 5A). A similar effect of PF was also found in WT mice (Fig. 5B). Total body fat mass of PFHFD and PFND Tg mice was similar, both groups displaying a significantly lower fat mass than that of HFD-fed Tg mice (Fig. 5C). For WT mice, limitation of caloric intake by PF reduced fat mass (Fig. 5C).
Figure 5.
PF abolished the phenotype of Tg mice fed with HFD. A and B, Body weight of Tg (A) and WT mice (B) with PF. Body weights were monitored every 4 wk (n = 5 of each group). C, Weight of fat mass at 16 wk of age (n = 5 of each group). D and E, Intraperitoneal glucose tolerance test at 16 wk of age (n = 4 of each group). Glucose response for Tg mice is shown in D. Glucose response for WT mice is shown in E. F and G, Intraperitoneal insulin tolerance test at 16 wk of age (n = 4 of each group). Glucose response for Tg mice is shown in F. Glucose response for WT mice is shown in G. Data are expressed as means ± sd. *, P < 0.05 and **, P < 0.01 vs. PFHFD group; †, P < 0.05 and ††, P < 0.01 vs. PFND group.
Next, we examined the effect of PF on glucose and ITT. During GTT, PFHFD and PFND Tg mice, and all three groups of WT mice exhibited significantly lower plasma glucose level compared with HFD-fed Tg mice, which showed an exaggerated response compared with the other groups (Fig. 5, D and E). ITT revealed a similar glucose response after insulin injection for PFHFD and PFND Tg mice compared with HFD-fed Tg mice which showed an impaired hypoglycemic response with a significantly higher blood glucose at 15 min after insulin treatment (Fig. 5, F and G). These findings strongly suggest that increased food intake caused the obesity, elevated fat mass, and insulin intolerance for Tg mice fed with HFD.
Hypothalamic neuropeptide expression in WT and Tg mice
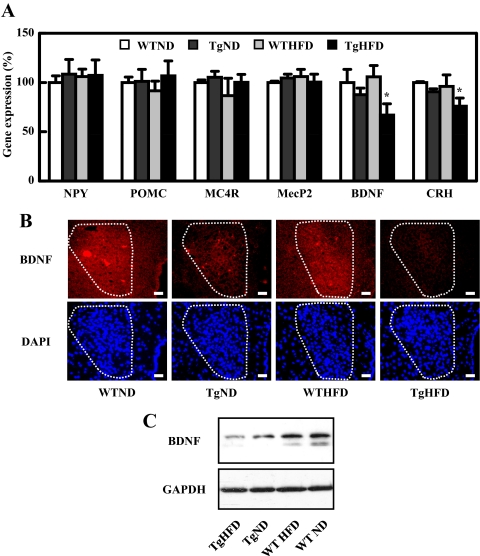
Based on the observation that HFD-fed Tg mice had increased feed intake and abnormal feeding behavior, we tested whether we can detect any dysregulation of their satiety center or feeding center. In the hypothalamus, one population of neurons expresses an orexigenic polypeptide, neuropeptide Y (NPY), whereas another expresses the anorexigenic polypeptide proopiomelanocortin (POMC) (15,16). Cleavage of POMC yields α-MSH, an anorexigenic neuropeptide. α-MSH binds to and activates melanocortin-4-receptor, a G-coupled receptor expressed by a number of neurons in the brain, including those in the paraventricular nucleus (16). We quantified the transcript level of different neuropeptides in the hypothalamus by qPCR, (Fig. 6A) and found that the mRNAs of NPY, POMC, and melanocortin-4-receptor in hypothalamus were similar among all groups of mice. Importantly, in HFD-fed Tg mice we detected a significant decrease in the expression of BDNF mRNA, a neuropeptide that regulates neuronal development as well as feeding behavior (17,18,19,20); there was, however, no difference in the expression level of MecP2, a gene that regulates BDNF in some circumstances (21). ND-fed Tg mice showed a small, insignificant reduction in BDNF mRNA level. We also detected a reduction in corticosterone-releasing hormone transcript level in Tg mice fed a HFD (Fig. 6A), a finding consistent with corticosterone-releasing hormone being a downstream target of BDNF (22). To determine whether relative immunoreactive BDNF was different at the peptide level, we performed immunofluorescence microscopy of the hypothalamus and found that immunoreactive BDNF peptide expression was reduced in the paraventricular region of the Tg mouse hypothalamus, especially in HFD-Tg mice (Fig. 6B). We also detected decreased expression in the ventromedial hypothalamic nucleus of HFD-Tg mice (data not shown). Western blotting using a BDNF antibody confirmed the down-regulation of immunoreactive BDNF in protein extracts from the whole hypothalamus in HFD-Tg mice compared with extracts from TgND, HFD-WT, and ND-WT mice (Fig. 6C).
Figure 6.
Hypothalamic neuropeptide expression. A, mRNA expression of neuropeptides that are involved in feeding behavior in hypothalamus at 16 wk of age. The relative expression of neuropeptides was expressed as the percentage of the ratio in WT mice fed with ND. Data are expressed as means ± sem. *, P < 0.05 vs. WT group. B, BDNF immunostaining (upper panel), and 4′,6-diamidino-2-phenylindole (DAPI, lower panel) for the paraventricular nucleus in the hypothalamus (highlighted by dotted lines) of 16-wk-old mice. Scale bar, 20 μm. C, Western blot for BDNF and GAPDH of hypothalamus from 16-wk-old mice.
Discussion
Over 20 million people in the U.S. are afflicted with diabetes, and more than 5 million individuals are living with AD. There is good epidemiological evidence that people with even mild type 2 diabetes are predisposed to developing AD and dementia (23). Our data indicate that when fed with HFD, Tg2576 mice, a Tg model for AD, exhibit obesity and insulin resistance, thus corroborating findings from previous studies (8,9,10). The Tg2576 mice showed significantly higher serum corticosterone level than WT littermates; however, there was no difference in serum corticosterone between HFD- and ND-fed Tg2576 mice (Fig. 2E), suggesting that elevated serum corticosterone level is not the cause of the insulin resistance in HFD-fed Tg2576 mice. On the other hand, HFD-fed Tg2576 mice displayed abnormal feeding behavior that led to a significantly increased food intake. These findings strongly suggest that increased caloric intake underlies the obesity and insulin resistance of Tg2576 mice fed with a HFD. This hypothesis is supported by the results of the PF experiment. The body fat masses of PFHFD and PFND Tg2576 mice were similar, both being significantly lower than that of HFD-fed Tg2576 mice. The impairment in the glucose response during GTT and ITT correlated mainly with the degree of diet-induced obesity in these groups of mice (Fig. 5). It is interesting that Cao et al. (24) found addition of 10% sucrose to the drinking water of APP/PS1 double Tg mice, a different mouse model of AD, caused exacerbation of memory impairment and increased amyloid-β protein. It is likely that the sucrose water treatment resulted in increased total caloric intake, leading to obesity and insulin resistance in these animals. Up to the age of 16 wk, we could not detect β-amyloid plaque using thioflavin-S staining, but readily detected Aβ condensation in the brains of Tg2576 mice. Because IDE is known to degrade insulin along with Aβ (4,5), in hyperinsulinemia, the binding of insulin to IDE may have interfered with Aβ degradation, leading to Aβ accumulation in the brain.
Interestingly, we detected a reduction in BDNF protein concentration in the hypothalamus of HFD-fed Tg2576 mice. BDNF is expressed in several energy balance centers in the hypothalamus, and is an integral component of central mechanisms that regulate learning and memory as well as satiety in mice (17,18,19,20). The increased Aβ in the brain of HFD-fed Tg2576 mice could be the consequence of down-regulated BDNF expression, which may lead to abnormal feeding behavior and increased food intake, resulting in severe insulin resistance in these animals. The mechanism by which Aβ down-regulates BDNF is unclear, although we have excluded changes in MecP2 transcript expression as a mechanism.
Insulin has pleiotropic biological effects in many different tissues. In the brain, it is involved in multiple regulatory pathways including neuronal survival, learning, control of energy homeostasis, and reproductive endocrinology, as well as food intake (25,26). Delivery of insulin to the brain has been reported to decrease expression of orexigenic NPY, increase anorexigenic POMC expression in the hypothalamus, and cause an anorexigenic effect, leading to weight loss (27,28). We found that the insulin concentration of the cerebrospinal fluid was also increased (data not shown) in HFD-fed Tg2576 mice. Perhaps because of the insulin resistance, the increase was apparently not sufficient to lead to increased food intake, which may be related to the insulin resistance of these animals. Recent studies have shown that Aβ inhibits insulin receptor autophosphorylation through competition for insulin binding to insulin receptor and through direct binding to the insulin receptor (29,30). Furthermore, it is reported that Aβ oligomers cause loss of neuronal surface insulin receptors and neuronal response to insulin (31). It is possible that insulin resistance caused by Aβ may affect neuronal activity and expression of BDNF in hypothalamus, which results in increased food intake and impaired feeding behavior in our mouse model.
Phillips et al. (32) found decreased transcript abundance of BDNF mRNA in the hippocampus of AD patients. They reported a 4-fold decrease of the in situ hybridization signal for BDNF in the dentate gyrus of AD compared with AD controls. Other reports have confirmed the finding, but it remains unclear whether decreased BDNF is a cause or an effect of local neuronal dropout (33). In our study, we also detected a significantly reduced BDNF expression in the brain of Tg2576 mice, even in the absence of detectable amyloid plaque burden or neuronal loss. Weight loss is a common problem among both community-dwelling and institutionalized older individuals with advanced AD and is associated with increased mortality and morbidity, as well as disease progression and poor quality of life (34,35). That may seem to contradict our finding that Tg2576 mice exhibited obesity and increased food intake. The cause of weight loss in AD population is thought to be multifactorial in origin, and functional and behavioral problems associated with AD make it difficult for individuals to consume adequate food intake. We found obesity and increased food intake on HFD-fed Tg2576 mice at 16 wk of age before the detection of any amyloid plaque. At this age, the mice would be model for early AD. Interestingly, although it has been reported that 44% of AD patients reported weight loss in the past 5 yr compared with 37% of the nondemented patients, a concomitant increase in food intake in 35% vs. 7%, respectively, was reported for the same populations (36). It is possible that decreased BDNF in AD brain is involved in modulating food intake in AD patients.
In summary, we showed that HFD-fed Tg2576 mice exhibit an age-dependent onset of peripheral insulin resistance associated with increased caloric intake. PF completely prevented their obesity and insulin intolerance, which indicates that increased caloric intake underlies this metabolic dysregulation. Tg2576 mice fed a HFD display higher basal insulin level from a young age, which is associated with increased Aβ in the brain. Increased Aβ in the brain via an unknown mechanism may have led to down-regulation of BDNF, an anorexigenic peptide, resulting in excess caloric intake, which may in turn worsen AD progression. Further characterization of the role of BDNF and caloric intake in AD and AD models may provide additional clues on the mechanisms involved in the progression and neuropathology of AD.
Footnotes
This work was supported by grants from the National Institutes of Health HL-51586 (to L.C.) and P30DK079638 for a Diabetes and Endocrinology Research Center, and by the Betty Rutherford Chair in Diabetes Research from St. Luke’s Episcopal Hospital and the T.T. and W.F. Chao Foundation.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 22, 2010
Abbreviations: Aβ, Amyloid β-peptide; AD, Alzheimer’s disease; BDNF, brain-derived neurotrophic factor; BSS, behavioral satiety sequence; GTT, glucose tolerance test; HFD, high-fat diet; IDE, insulin-degrading enzyme; ITT, insulin tolerance test; ND, normal diet; NPY, neuropeptide Y; Tg, transgenic; PF, pair feeding; POMC, proopiomelanocortin; qPCR, quantitative RT-PCR; WT, wild type.
References
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, Palumbo PJ 1997 Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 145:301–308 [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM 1999 Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 53:1937–1942 [DOI] [PubMed] [Google Scholar]
- Bertram L, Blacker D, Mullin K, Keeney D, Jones J, Basu S, Yhu S, McInnis MG, Go RC, Vekrellis K, Selkoe DJ, Saunders AJ, Tanzi RE 2000 Evidence for genetic linkage of Alzheimer’s disease to chromosome 10q. Science 290:2302–2303 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ 2001 Clearing the brain’s amyloid cobwebs. Neuron 32:177–180 [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Folstein MF 2006 Insulin, insulin-degrading enzyme and amyloid-β peptide in Alzheimer’s disease: review and hypothesis. Neurobiol Aging 27:190–198 [DOI] [PubMed] [Google Scholar]
- Kuusisto J, Koivisto K, Mykkänen L, Helkala EL, Vanhanen M, Hänninen T, Kervinen K, Kesäniemi YA, Riekkinen PJ, Laakso M 1997 Association between features of the insulin resistance syndrome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ 315:1045–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft S, Asthana S, Schellenberg G, Cherrier M, Baker LD, Newcomer J, Plymate S, Latendresse S, Petrova A, Raskind M, Peskind E, Lofgreen C, Grimwood K 1999 Insulin metabolism in Alzheimer’s disease differs according to apolipoprotein E genotype and gender. Neuroendocrinology 70:146–152 [DOI] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM 2004 Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J 18:902–904 [DOI] [PubMed] [Google Scholar]
- Pedersen WA, Flynn ER 2004 Insulin resistance contributes to aberrant stress responses in the Tg2576 mouse model of Alzheimer’s disease. Neurobiol Dis 17:500–506 [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ho L, Wang J, Qin W, Festa ED, Mobbs C, Hof P, Rocher A, Masur S, Haroutunian V, Pasinetti GM 2005 Connective tissue growth factor (CTGF) expression in the brain is a downstream effector of insulin resistance-associated promotion of Alzheimer’s disease β-amyloid neuropathology. FASEB J 19:2081–2082 [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G 1996 Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274:99–102 [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH 2002 The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer’s disease. J Neurosci 22:1858–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha PK, Kojima H, Martinez-Botas J, Sunehag AL, Chan L 2004 Metabolic adaptations in the absence of perilipin: increased β-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem 279:35150–35158 [DOI] [PubMed] [Google Scholar]
- Halford JC, Wanninayake SC, Blundell JE 1998 Behavioral satiety sequence (BSS) for the diagnosis of drug action on food intake. Pharmacol Biochem Behav 61:159–168 [DOI] [PubMed] [Google Scholar]
- Ramos EJ, Meguid MM, Campos AC, Coelho JC 2005 Neuropeptide Y, α-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition 21:269–279 [DOI] [PubMed] [Google Scholar]
- Millington GW 2007 The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr Metab 4:18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons WE, Mamounas LA, Ricaurte GA, Coppola V, Reid SW, Bora SH, Wihler C, Koliatsos VE, Tessarollo L 1999 Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 96:15239–15244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernie SG, Liebl DJ, Parada LF 2000 BDNF regulates eating behavior and locomotor activity in mice. EMBO J 19:1290–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios M, Fan G, Fekete C, Kelly J, Bates B, Kuehn R, Lechan RM, Jaenisch R 2001 Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol 15:1748–1757 [DOI] [PubMed] [Google Scholar]
- Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF 2003 Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci 6:736–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Chang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME 2003 Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 302:885–889 [DOI] [PubMed] [Google Scholar]
- Givalois L, Naert G, Rage F, Ixart G, Arancibia S, Tapia-Arancibia L 2004 A single brain-derived neurotrophic factor injection modifies hypothalamo-pituitary-adrenocortical axis activity in adult male rats. Mol Cell Neurosci 27:280–295 [DOI] [PubMed] [Google Scholar]
- Xu W, Qiu C, Winblad B, Fratiglioni L 2007 The effect of borderline diabetes on the risk of dementia and Alzheimer’s disease. Diabetes 56:211–216 [DOI] [PubMed] [Google Scholar]
- Cao D, Lu H, Lewis TL, Li L 2007 Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J Biol Chem 282:36275–36282 [DOI] [PubMed] [Google Scholar]
- Gerozissis K, Rouch C, Lemierre S, Nicolaidis S, Orosco M 2001 A potential role of central insulin in learning and memory related to feeding. Cell Mol Neurobiol 21:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Belgardt BF, Brüning JC 2006 Central insulin action in energy and glucose homeostasis. J Clin Invest 116:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC 2002 The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci 22:9048–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhorst U, de Fries D, Steingrueber HJ, Scherbaum WA 2004 Insulin and the CNS: effects on food intake, memory, and endocrine parameters and the role of intranasal insulin administration in humans. Physiol Behav 83:47–54 [DOI] [PubMed] [Google Scholar]
- Xie L, Helmerhorst E, Taddei K, Plewright B, Van Bronswijk W, Martins R 2002 Alzheimer’s β-amyloid peptides compete for insulin binding to the insulin receptor. J Neurosci 22:RC221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M, Mehta T, Selkoe DJ 2007 Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem 282:33305–33312 [DOI] [PubMed] [Google Scholar]
- Zhao WQ, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL 2008 Amyloid β oligomers induce impairment of neuronal insulin receptors. FASEB J 22:246–260 [DOI] [PubMed] [Google Scholar]
- Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW 1991 BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 7:695–702 [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Chauhan NB 2000 Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res Brain Res Rev 33:199–227 [DOI] [PubMed] [Google Scholar]
- Wolf-Klein GP, Silverstone FA 1994 Weight loss in Alzheimer’s disease: an international review of the literature. Int Psychogeriatr 6:135–142 [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Shea S, Mayeux R 2002 Caloric intake and the risk of Alzheimer disease. Arch Neurol 59:1258–1263 [DOI] [PubMed] [Google Scholar]
- Wolf-Klein GP, Silverstone FA, Levy AP 1992 Nutritional patterns and weight change in Alzheimer patients. Int Psychogeriatr 4:103–118 [DOI] [PubMed] [Google Scholar]