Abstract
Pancreatic islet cells use neurotransmitters such as l-glutamate to regulate hormone secretion. We determined which cell types in mouse pancreatic islets express ionotropic glutamate receptor channels (iGluRs) and describe the detailed biophysical properties and physiological roles of these receptors. Currents through iGluRs and the resulting membrane depolarization were measured with patch-clamp methods. Ca2+ influx through voltage-gated Ca2+ channels and Ca2+-evoked exocytosis were detected by Ca2+ imaging and carbon-fiber microamperometry. Whereas iGluR2 glutamate receptor immunoreactivity was detected using specific antibodies in immunocytochemically identified mouse α- and β-cells, functional iGluRs were detected only in the α-cells. Fast application of l-glutamate to cells elicited rapidly activating and desensitizing inward currents at −60 mV. By functional criteria, the currents were identified as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptors. They were activated and desensitized by AMPA, and were activated only weakly by kainate. The desensitization by AMPA was inhibited by cyclothiazide, and the currents were blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX). Islet iGluRs showed nonselective cation permeability with a low Ca2+ permeability (PCa/PNa = 0.16). Activation of the AMPA receptors induced a sequence of cellular actions in α-cells: 1) depolarization of the membrane by 27 ± 3 mV, 2) rise in intracellular Ca2+ mainly mediated by voltage-gated Ca2+ channels activated during the membrane depolarization, and 3) increase of exocytosis by the Ca2+ rise. In conclusion, iGluRs expressed in mouse α-cells resemble the low Ca2+-permeable AMPA receptor in brain and can stimulate exocytosis.
AMPA-type glutamate receptors control hormone secretion from pancreatic α-cells.
Glutamate, the predominant excitatory neurotransmitter in the mammalian central nervous system (CNS), mediates fast synaptic transmission by acting on ionotropic glutamate receptors (iGluRs) (1,2). The endocrine pancreas is one of the very few places outside the CNS where glutamate-mediated signaling is implicated (3,4,5). In the pancreatic islet of Langerhans, l-glutamate together with inhibitory γ-aminobutyric acid (GABA) are proposed as intercellular paracrine signals that regulate the hormone secretion involved in glucose homeostasis (6).
Pancreatic islets have all components required for glutamatergic transmission: glutamate sources, receptors, and clearance mechanisms. The glucagon-secreting α-cells express vesicular glutamate transporter subtypes 1 and 2, accumulate l-glutamate into large dense-core granules containing glucagon, and secrete both of them in parallel in low-glucose conditions (7). The secreted extracellular l-glutamate is sequestered by high-affinity glutamate/aspartate transporters in non-β-cells (8,9). Nevertheless, the expression of iGluRs in islets seems complicated and even controversial (4). α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)-type iGluRs have been reported both in dissociated glucagon-secreting α-cells and in insulin-secreting β-cells (10,11,12,13,14), and their stimulation enhanced the secretion of glucagon and insulin in intact rat islets and perfused pancreas (12,13,15,16,17). Somatostatin-secreting δ-cells of rat expressed a newly identified splice variant of AMPA receptors that promotes somatostatin release (18). Expression of kainate-type iGluRs was reported in dissociated rat α-cells, δ- cells, and islets (10,12,13), but their functional roles have not been examined. N-methyl-d-aspartic acid (NMDA)-type iGluR immunoreactivity was also detected in β-cells and rat islets (13,14), and NMDA receptor activation elicited insulin secretion (12). In contrast, l-glutamate inhibited glucagon secretion from rat islets via metabotropic GluR subtypes such as metabotropic GluR2, 4, and 8 that couple to Gi/Go G proteins (19,20).
In sum, it has been reported that several subtypes of iGluRs exist in pancreatic islet cells and that their activation promotes secretion of islet hormones. However, many of these studies were carried out in unidentified and mixed islet cells, intact islets, or whole pancreas, making mechanistic interpretations difficult. Does l-glutamate act directly on the cell type of interest or indirectly via paracrine signaling from other cells? Therefore, it is necessary to test for functional iGluRs using single isolated cells. Here, we identified cell types based on size, hormone-specific antibodies, single-cell RT-PCR, and investigated iGluR expression. In addition, we investigated the electrophysiological and pharmacological properties and the effects of iGluR activation on intracellular free Ca2+ concentration ([Ca2+]i) and on exocytosis of secretory vesicles using single-cell techniques.
Materials and Methods
Cell preparation
The preparation of islets and single cells was previously reported (21). Animal care followed the University of Washington Animal Medicine guidelines. Islets of Langerhans were isolated from 5- to 10-wk-old male BALB/c mice euthanized by CO2. Isolated cells plated on coverslips coated with poly-l-ornithine were cultured in RPMI 1640 culture medium for 1–2 d before use.
Solutions and chemicals
For whole-cell voltage-clamp experiments, Na+-rich external solution contained (in mmol/liter): 135 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 3.3 glucose, 10 TEA, and 10 HEPES (pH 7.3 with NaOH). Ca2+-rich external solution contained: 100 CaCl2, 1 MgCl2, 3.3 glucose, 10 TEA, and 5 HEPES [pH 7.3 with Ca(OH)2]. Patch pipettes were filled with: 125 CsCl, 4 NaCl, 1 CaCl2, 1 MgCl2, 5 EGTA, 2 MgATP, 15 TEA, and 5 HEPES (pH 7.3 with CsOH). For current-clamp experiments, K+-rich internal solution contained: 140 KCl, 4 NaCl, 1 CaCl2, 1 MgCl2, 5 EGTA, 2 MgATP, and 5 HEPES (pH 7.3 with KOH). For [Ca2+]i measurements and amperometric experiments, the external solution contained: 140 NaCl, 5.6 KCl, 2.5 CaCl2, 1 MgCl2, 3.3 glucose, and 10 HEPES (pH 7.3 with NaOH). In Na+-free solutions, NaCl was replaced by equimolar N-methyl-D-glucamine (NMDG). Fura 2-AM (Molecular Probes, Eugene, OR), R,S-AMPA, kainate, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and cyclothiazide (Tocris, Ellisville, MO) were freshly dissolved before experiments. Culture medium and serum were from Invitrogen (Carlsbad, CA) and all other chemicals from Sigma (St. Louis, MO).
Immunocytochemistry
Islet cells were fixed with 2% paraformaldehyde in a Na+-rich solution, permeabilized with 0.2% Triton X-100, and treated with a blocking solution (Pierce, Rockford, IL) to reduce nonspecific binding of antibodies (Fig. 1). Cells were incubated with primary antibodies at 4 C overnight. After washing, cells were incubated with secondary antibodies at 22 C for 1 h. Antihormone antibodies used were as follows: antiinsulin (1:75 dilution; catalog no. 250-2788, Ventana, Tucson, AZ), antiglucagon (1:75 dilution; catalog no. A0565, DakoCytomation, Carpinteria, CA), and antisomatostatin (1:100 dilution; catalog no. sc-13099, Santa Cruz Biotechnology, Santa Cruz, CA); secondary antibodies for antiinsulin antibody [fluorescein isothiocyanate (FITC)-conjugated goat antimouse, 1:100 dilution; catalog no. 115-095-003, Jackson ImmunoResearch, West Grove, PA; or Alexa 488, 1:1000 dilution; catalog no. A11008, Molecular Probes] and for antiglucagon and antisomatostatin antibody [tetramethylrhodamine B isothiocyanate (TRITC)-conjugated goat antirabbit, 1:100 dilution; catalog no. 111-025-003, Jackson ImmunoResearch]. The coverslips were mounted on slides using Gel/Mount (Biomeda, Foster city, CA). To determine which cell types express iGluR, islet cells were first tested for kainate-induced [Ca2+]i response and then stained with hormone-specific antibodies as in Fig. 2. A coverslip with etched alphanumeric coordinates (Bellco, Vineland, NJ) was used to match the cells in the two experiments. Images were collected using a Deltavision SA3.1 deconvolution microscope (Applied Precision, Issaquah, WA) or a Leica SP1/MP confocal microscope (Leica Microsystems, Inc., Bannockburn, IL).
Figure 1.
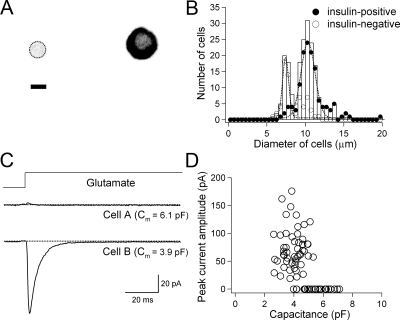
Preferential expression of iGluRs in small islet cells. A, Isolated single islet cells were immunostained with antiinsulin antibody. A low magnification image is present to illustrate the contrast between an insulin-negative (left) and an insulin-positive cell (right) in the same visual field. Shown as an inverted grayscale image (fluorescence is dark). The cell boundary of the insulin-negative cell is shown with broken line. Scale bar, 5 μm. Diameter of cells was measured after digital zooming. B, Histogram of cell diameters fitted by two Gaussian distributions (smooth lines) (n = 202). C, Time course of ionic current in two cells with different whole-cell capacitance in response to rapid application of 1 mmol/liter l-glutamate at a holding potential of −60 mV. D, Peak current amplitude activated by 1 mmol/liter l-glutamate plotted against whole-cell capacitance (n = 76).
Figure 2.
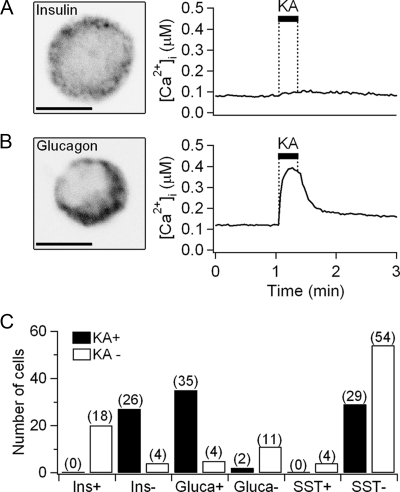
Expression of iGluRs in immunocytochemically identified cells. A, An image of a single cell immunostained with antiinsulin antibody (scale bar, 5 μm) and the time course of Ca2+ response to 0.5 mmol/liter kainate (KA) for 20 sec. The cell was kainate unresponsive, relatively small, and insulin-positive. Insulin granules are labeled as punctate structures outside the nucleus. B, An image of another cell immunostained with antiglucagon antibody and its Ca2+ response to kainate. The cell was kainate responsive, small, and glucagon-positive. C, Summary bar graph showing the number of cells responding to kainate stimulation (KA+, black) or not responding (KA−, white). All the cells were sorted by their immunocytochemical identification as positive (+) or negative (−) for the indicated peptides. The numbers in parentheses indicated the total number of tested single cells.
Single-cell RT-PCR
After membrane capacitance (Cm) measurement, each cell’s cytoplasm was sucked into the recording patch pipette and stored in RT-PCR reaction buffer included in a one-step RT-PCR kit (QIAGEN, Valencia, CA) (Fig. 3). Intron-spanning specific mouse glucagon primer pairs were as described by Vignali et al. (forward 5′-GACTTCCCAGAAGAAGTCGCCAT-3′; reverse_ 5′-CTACGGTTACCAGGTGGTCATGT-3′) (22). Two samples served as negative controls: 1) a minus-RT control where an aliquot of without reverse transcriptase was used for RT reaction, and 2) a minus-template PCR, containing all of the reaction components but a single cell. For the amplification of insulin messengers, intron-spanning specific mouse primer pairs were used (forward primer_5′-CAGCAAGCAGGTCATTGTTT-3′; reverse_5′-CAGTAGTTCTCCAGCTGGTAGA-3′) (22).
Figure 3.
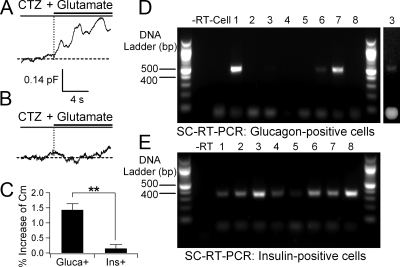
Expression of iGluRs in cells identified with single-cell RT-PCR. A and B, Representative Cm traces in response to 100 μm cyclothiazide (CTZ) and 1 mm l-glutamate from a single identified α (A) and β (B) cell. After the Cm measurement, the cell type was identified using single-cell RT-PCR with either glucagon (Gluca+)- or insulin (Ins+)-specific primers (lane 6 in panel D and lane 2 in panel E, respectively). C, Percent increase of Cm from α (n = 4) and β-cells (n = 6). Data are shown as mean ± sem. **, P < 0.01. D, Single-cell RT-PCR for glucagon expression. Each cell’s cytoplasm was harvested after capacitance measurement using glucagon primer pairs. For negative controls, reverse transcriptase (−RT) or cell content (−Cell) was omitted. Glucagon PCR products were found in only four cells (1,3,6,7). A second image of lane 3 is shown at the end with enhanced contrast. Lane 8 was a cell whose cytoplasm was collected for single-cell RT-PCR without Cm measurement. E, Single-cell RT-PCR for insulin expression. Six cells (lane 1–6) showed the insulin PCR products after Cm measurement. Lanes 7 and 8 are from two single cells whose cytoplasm was collected for single-cell RT-PCR without Cm measurement.
Electrophysiology
Gigaseal clamp measurements were performed in the whole-cell configuration (23). After formation of the whole-cell configuration, negative pressure (∼−200 mbar) was applied to the pipette for approximately 2 min to attract the cell nucleus to the glass tip. The access resistance increased but by less than 25 mΩ. With the support of the nucleus, single islet cells could be detached from the glass bottom to permit faster application of agonists. The optimal pipette size for lifting cells was 3–5 mΩ when filled with the pipette solution. For single-channel recordings, excised outside-out patches were pulled out after the formation of the whole-cell configuration. For fast agonist application experiments, a double-barreled perfusion pipette was fabricated from θ-glass tubing and attached to a piezo-electric element (ceramic multilayer bender 5 from Noliac, Kvistgaard, Denmark). The solution exchange time (10–90%) measured electrically with an open patch-pipette tip during a switch from 100% Na+ to 10% Na+ solution was about 0.5–1 msec. For the experiments of Figs. 2, 4C, 5, and 6 and Supplemental Fig. 7 (see Supplemental Figs. 1–7 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org), solutions were applied through a multibarreled perfusion system that had a solution exchange time less than 0.5 sec (24). Membrane currents were recorded with an EPC-9 amplifier (HEKA Elektronik, Lambrecht/Pfalz, Germany), filtered at 1 kHz with an 8-pole, low-pass Bessel filter, and sampled at 5 kHz, unless noted. Cm before and after glutamate stimulation was measured with the Lindau-Neher technique implemented as the “sine + dc” mode of the software lock-in extension of Pulse (HEKA Elektronik), in which a 1-kHz, 25-mV sinusoidal voltage stimulus (50 mV, peak to peak) is superimposed onto a holding potential of −80 mV.
Figure 4.
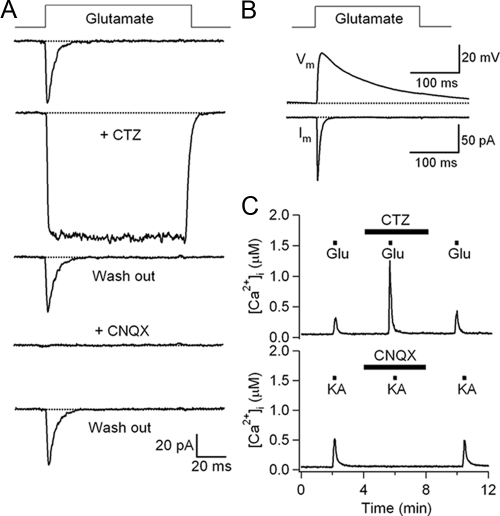
Pharmacological properties of AMPA receptors. A, Time course of currents evoked by 1 mmol/liter l-glutamate in the absence or presence of 100 μmol/liter cyclothiazide (CTZ) or 10 μmol/liter CNQX in the same cell. Membrane potential was held at −60 mV. B, Change of membrane potential induced by a 200-msec pulse of 1 mmol/liter l-glutamate using current-clamp recording mode. The peak depolarization was 42 mV in this experiment (upper trace). Current response was measured in the voltage-clamp recording mode at −60 mV from the same cell (lower trace). C, The [Ca2+]i response evoked by 0.5 mmol/liter l-glutamate was potentiated by inhibition of AMPAR desensitization using 100 μmol/liter cyclothiazide (n = 9). Block of AMPAR by 10 μmol/liter CNQX abolished [Ca2+]i response to 0.5 mmol/liter kainate (n = 3).
Figure 5.
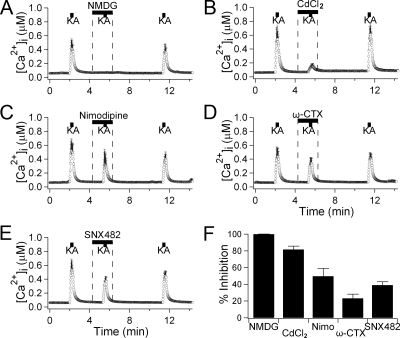
Effect of extracellular Na+ removal and of Ca2+ channel blockers on kainate-induced [Ca2+]i response. A, [Ca2+]i responses to 0.5 mmol/liter kainate (KA) for 10 sec in the presence and absence of Na+ (n = 5). The 135 mm extracellular Na+ was replaced by equimolar NMDG+ to prevent depolarization by Na+ influx through iGluRs. The [Ca2+]i trace in this figure is the average for all measured cells, and has error bars. B–E, [Ca2+]i with several Ca2+ channel blockers. Kainate-evoked [Ca2+]i increase was measured in the presence of 200 μm CdCl2 as a nonselective blocker of Ca2+ channels (n = 9), 10 μm nimodipine (“Nimo”) for blocking L-type Ca2+ channels (n = 12), 2 μmol/liter ω-conotoxin GIVA (“ω-CTX”) for N-type Ca2+ channels (n = 18), and 100 nmol/liter SNX482 for R-type Ca2+ channels (n = 11). F, Percent inhibition of kainate-induced [Ca2+]i peaks by each condition. Data are shown as mean ± sem.
Figure 6.
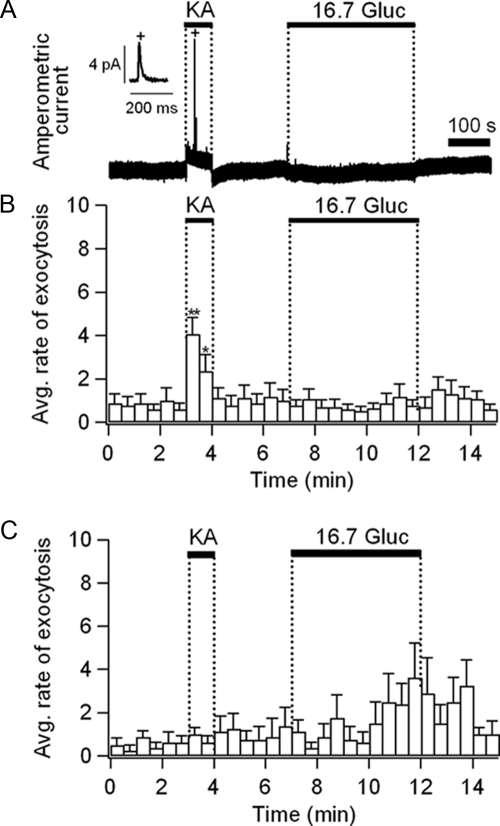
Increase of exocytosis by AMPA-receptor activation in single islet cells. A, Raw time course of amperometric current obtained in kainate (1 mmol/liter, 1 min) or glucose (16.7 mmol/liter, 5 min). Inset, One exocytotic event, marked with an asterisk, is displayed on an expanded time scale to show typical quantal exocytotic release of oxidizable molecules. B and C, Averaged rate of exocytosis for small islet cells (B, n = 17) and large islet cells (C, n = 7). Data are expressed as mean ± sem. For the transient kainate response, significance levels are indicated as *, P < 0.01 and **, P < 0.0005. These experiments were performed at 35 C, because exocytosis from β-cells is reduced at lower temperatures (50).
Ca2+ measurement and amperometry
Cytoplasmic free Ca2+ concentration ([Ca2+]i) was monitored with Fura 2 Ca2+-sensitive dye. Cells were loaded with 2 μmol/liter Fura 2-AM in a Na+-rich solution containing 3 or 5 mmol/liter glucose for 30 min at 37 C (25). The dye was excited alternately at 340 and 380 nm, and the fluorescence signals were recorded every 1 sec at 510 nm using a charge-coupled device camera (Roper, Tucson, AZ). Most Ca2+ measurements were done at approximately 22 C (with the exception of those in Supplemental Fig. 7). For measuring exocytosis, cells were preincubated in culture medium that was supplemented with 2 mmol/liter serotonin for 4–16 h (26). The carbon-fiber amperometric electrode (27) was connected to an EPC-9 patch clamp amplifier and held at 600 mV. Quantal serotonin release from individual granules was detected as single spikes of oxidation current by the electrode. All amperometric recordings (Fig. 6) were performed at 33–36 C.
Data analysis
Membrane current and voltage traces shown in the figures are averages of two to five single traces. Desensitization time constants of the AMPAR-mediated component were determined by fitting the decay phases with a single-exponential function. Concentration-response curves were fitted with a Hill function:
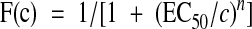 |
1 |
where c, EC50, and n denote concentration of agonists, half-maximal effective concentration, and Hill coefficient, respectively. Data points of current-voltage (I-V) relations were fitted with a second-order polynomial, from which the reversal potentials were determined by interpolation. Rectification index was calculated by dividing the conductance at +40 mV by that at −40 mV. Ca2+ permeability ratio was calculated from the modified Goldman-Hodgkin-Katz voltage equation (28):
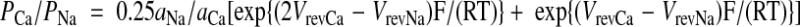 |
2 |
where aNa and aCa represent the activities of Na+ and Ca2+ in the extracellular solutions, respectively. R, T, and F have their conventional thermodynamic meaning. Reversal potentials measured under high Na+ or Ca2+ external solutions (VrevNa and VrevCa) were corrected for liquid junction potentials of 4.5 and 10.3 mV, respectively, in Supplemental Fig. 5D. Data were analyzed in Igor Pro (WaveMetrics, Lake Oswego, OR). All averaged values were given as mean ± sem. Student’s t test was used for statistical tests, and P values less than 0.05 were considered statistically significant.
Results
α-Cells respond to glutamate
Using dissociated islet cells, we determined the cell-size dependence of insulin expression and the cell-size dependence of functional responses to iGluR agonists. Insulin-secreting β-cells tend to be larger than glucagon-secreting α-cells or somatostatin-secreting δ-cells in purified rat islet preparations (29). We measured the size distribution of mouse insulin-positive and insulin-negative cells using antiinsulin antibody (Fig. 1A). These two categories of cells fell into diameter classes that could be approximated by two Gaussian distributions (Fig. 1B). Insulin-positive cells had larger average diameters (10.7 ± 0.2 μm, n = 134 cells) than insulin-negative cells (8.3 ± 0.2 μm, n = 68). The two distributions overlapped in the 7- to 10-μm range. Electrical responses of iGluRs were assessed by applying 1 mmol/liter l-glutamate rapidly to single patch-clamped cells lifted from the glass bottom (Fig. 1C; also, see Materials and Methods), and cell size was estimated as membrane electrical capacitance, Cm. l-glutamate consistently evoked transient inward currents in small cells (53 tested cells with Cm ≤ 4.0) but not in large cells (23 tested cells with Cm ≥ 5.5). Inward currents are shown as downward deflections by convention. For rough comparison with the diameter measurements, a cell with 4 pF of Cm would have a plasma membrane surface area of 400 μm2, and if this were a smooth perfect sphere, the diameter would be 11.3 μm, but a real, somewhat convoluted cell would be smaller. Figure 1D plots the peak current amplitudes evoked by l-glutamate in different cells showing peak currents ranging from approximately 10 pA to approximately 150 pA. Thus, the glutamate-responsive cells are small, whereas the immunocytochemistry in separate experiments shows that insulin-containing islet cells are large.
Because of some overlap of size distributions, it seemed worthwhile to test for iGluR function and hormone content in the same individual cells. In this case, function was tested using calcium imaging in a field of islet cells. The cells were challenged with 0.5 mm kainate to measure [Ca2+]i responses, and then stained with an antihormone antibody (Fig. 2A, see also Fig. 4C for [Ca2+]i rise mediated by kainate-activated iGluR). No insulin-positive β-cells (n = 0/18) responded to kainate (Fig. 2C). As expected, some cells with diameters of 7–10 μm were identified as β-cells (Fig. 1B), and they were not responsive to kainate (Fig. 2A). Thus, in this middle diameter range, the identification of cell types solely based on cell size would not be reliable. Most of the insulin-negative, non-β-cells responded to kainate (26/30 cells). Among the islet cells that stained with antiglucagon antibody after the [Ca2+]i measurement, most showed a positive response to kainate (35/39 glucagon-positive cells). The exact identity of two glucagon-negative cells out of 13 cells is not known. They could represent a small percentage of non-α-cells expressing iGluRs or α-cells that stained weakly with the antiglucagon antibody. Finally, islet cells were stained with antisomatostatin antibody. When [Ca2+]i measurements and immunocytochemistry were done in 87 cells in parallel, only four cells were positive to antisomatostatin antibody. None of these four cells responded to kainate. Of the 83 somatostatin-negative cells, 29 were responsive to kainate. We note that the proportions of each cell type in dissociated single-cell cultures was quite variable between batches. We suggest that this is an artifact of detachment of some larger β-cells from the glass chips during the transfers from the culture dish to the measuring chamber and during processing for immunohistochemistry.
In addition to size distribution and immunocytochemistry, we also identified hormone expression of single cells by single-cell RT-PCR. We paired this assay with tests for functional iGluRs in the same individual cell. In this experiment, functional activity was gauged by measurements of Cm changes. The reasoning was as follows. Islet iGluRs have a small but measurable Ca2+ permeability (see below), and activated receptors would then raise Ca2+ and induce exocytosis. If we observed an increase of Cm after agonist addition that would indicate a plasma membrane surface area increase from addition of secretory vesicles (exocytosis) in response to Ca2+ influx through activated receptors. For this test, we maximized this Ca2+ influx by eliminating iGluR desensitization with cyclothiazide and by holding the membrane at a very negative potential −80 mV. Indeed, there was an increase of Cm in some cells (Fig. 3A), mainly small ones, and not in other cells (Fig. 3B). Next, the cytoplasm of each cell was harvested into the patch pipette for hormone identification by single-cell RT-PCR with either glucagon- or insulin-specific primers. Glucagon PCR products were detected in four tested cells (lanes 1, 3, 6, and 7 in Fig. 3D), and insulin PCR products were detected in six other tested cells (lanes 1–6 in Fig. 3E). The capacitance change of the four glucagon-positive cells was significantly larger than that of six insulin-positive cells (Fig. 3C).
We also looked for iGluR2 immunoreactivity in immunohistochemically identified mouse islet cells (Supplemental Fig. 1). Unexpectedly, but similar to a previous study (10) in rat islets, both the mouse α- and β-cells, but not the δ-cells, showed immunoreactivity for iGluR2. Thus, whereas the results in Figs. 1 and 2 reveal functional iGluRs only in α-cells, immunostaining suggests that there is nonfunctional iGluR2 protein in β-cells as well.
α-Cells express AMPA receptors
We analyzed the basic functional characteristics of islet iGluRs and found that they corresponded to the AMPA subtype as shown in the supplemental figures. For these, studies l-glutamate, AMPA, and kainate were applied rapidly to islet cells (Supplemental Fig. 2A). Inward currents activated by 100-msec pulses of 1 and 10 mmol/liter l-glutamate or AMPA rose to a peak within 2 msec and desensitized within 10 msec. The average exponential time constant for desensitization was 5.1 ± 0.1 msec (10 mmol/liter l-glutamate; n = 10). To test whether the agonist application might be retarded by the whole cell in the front of a patch pipette, we also measured kinetics using outside-out patches (Supplemental Fig. 3). The desensitization time constant obtained from ensemble averages of these single-channel recordings was 6.1 ± 0.3 msec (n = 6). The excised-patch experiments indicated a single-channel current of 0.68 ± 0.03 pA (n = 12 from three different patches) at −60 mV and a chord conductance of 11.3 pS.
As is characteristic of AMPA receptors, the currents with kainate were smaller and showed primarily a nondesensitizing component. The concentration dependence of the evoked currents was estimated for each agonist (Supplemental Fig. 2, B–D). The half-maximal activating concentrations (EC50) for activation of peak current were similar: 720 μmol/liter for AMPA, 841 μmol/liter for l-glutamate, and 882 μmol/liter for kainate. Steady-state current was evoked with an EC50 value of 530 μmol/liter kainate.
The I-V relation for l-glutamate-activated peak currents in Na+-rich external solution showed a reversal potential at 1.2 ± 0.7 mV (n = 5), indicating an approximately equal permeability for the extracellular Na+ and intracellular Cs+ ions (Supplemental Fig. 4). Similarly, the reversal potentials measured with AMPA and kainate were 0.1 ± 2.1 mV (n = 7) and −1.9 ± 1.0 mV (n = 9), respectively. The I-V relation was linear when iGluRs were activated with glutamate or AMPA but strongly outwardly rectifying when activated by kainate. When external monovalent cations were entirely replaced by Ca2+, the reversal potential for glutamate-activated peak current shifted to very negative values, −60.1 ± 1.1 mV (n = 10, Supplemental Fig. 5C) (corrected for liquid junction potentials). From Eq. 2, the relative Ca2+ permeability of iGluRs was therefore low (PCa/PNa = 0.16 ± 0.01; Supplemental Fig. 4D, n = 10) compared with highly Ca2+ permeable iGluRs (30).
To address again whether AMPA- or kainate-type iGluRs contribute to the current activated by l-glutamate, we investigated the effect of cyclothiazide, a blocker of desensitization with high specificity for AMPA-type over kainate-type iGluRs (30,31). Cyclothiazide reversibly blocked the desensitization of iGluR currents in pancreatic islet cells (Fig. 4A) and doubled the peak current (186 ± 10% compared with control, n = 7). Cyclothiazide also potentiated the increase of intracellular free Ca2+ concentration ([Ca2+]i) evoked by 1 mmol/liter l-glutamate (Fig. 4C). The [Ca2+]i increase evoked by 1 mmol/liter l-glutamate together with 100 μm cyclothiazide was 720 ± 186 nmol/liter (n = 9), whereas the change with l-glutamate alone was only 94 ± 18 nmol/liter. Currents evoked by 1 mmol/liter l-glutamate were completely and reversibly blocked by 10 μm CNQX, an antagonist of AMPA and kainate-type iGluRs (n = 3, Fig. 4A). Similarly, CNQX also reduced [Ca2+]i responses evoked by 0.5 mmol/liter kainate by up to 96% (n = 16, Fig. 4C). We conclude that the kainate responses we see in islet cells are due to its well-known nondesensitizing activation of AMPA receptors rather than to activation of true kainate receptors.
Activation of AMPAR depolarizes the membrane and activates voltage-gated Ca2+ channels
In neurons, iGluR-mediated inward currents depolarize the cell membrane to produce excitatory postsynaptic potentials. The same occurred in islet cells. Recording in current-clamp mode (Fig. 4B), islet cells had a resting membrane potential of −67 ± 5 mV (n = 8) and depolarized transiently by 27 ± 3 mV (n = 8) upon application of 1 mmol/liter l-glutamate. The membrane potential decayed to the resting level with a time constant of 68 ± 7 msec (n = 8, much longer than the decay time constant of iGluR currents (5.7 ± 0.1 msec, n = 9).
Such membrane depolarizations should also activate voltage-gated Ca2+ channels as in neurons. Indeed, both kainate and glutamate increased [Ca2+]i levels (Fig. 4C). As expected, the response to the nondesensitizing agonist kainate was larger than that with desensitizing l-glutamate and AMPA. Kainate triggered detectable [Ca2+]i rises in 84% (366 of 435 cells), whereas l-glutamate and AMPA triggered [Ca2+]i rises in 34% (71 of 209 cells) and 56% (126 of 226 cells) of cells, respectively. The experiments presented in Fig. 5 further show that these Ca2+ elevations are primarily due to opening of voltage-gated Ca2+ channels by the prolonged depolarization. When the depolarization was prevented by replacing extracellular Na+ with NMDG (Fig. 5A), which is not measurably permeant in AMPA receptors (32,33), no [Ca2+]i increases were evoked by kainate (0.5 mmol/liter, n = 5). When all subtypes of voltage-gated Ca2+ channels were blocked with nonselective CdCl2, the [Ca2+]i rises were reduced by 84% (n = 9, Fig. 5B). The residual small Ca2+ signal appears to be mediated by Ca2+ influx through the iGluRs themselves. The [Ca2+]i rise was partially reduced by more selective blockers of L-, N-, and R-subtype Ca2+ channels, suggesting involvement of several voltage-activated Ca2+ channel types (Fig. 5, C–F).
Activation of AMPA receptors triggers exocytosis in α-cells
Pancreatic islet cells secrete endocrine hormones and neurotransmitters when [Ca2+]i increases (5,6). Therefore, we tested whether the [Ca2+]i rises evoked by AMPA receptor activation would be sufficient to trigger exocytosis. As noted in Fig. 1, the size distributions of β- and non-β-cells overlap, especially in the diameter range between 7 and 10 μm (Fig. 1). Therefore, we used cells smaller than 7 μm for non-β-cells and cells larger than 10 μm for β-cells. Based on these criteria, the probability of correct cell identification was more than 90% from the size distribution shown in Fig. 1B. To measure exocytosis with carbon-fiber amperometry, we loaded islet cells with exogenous oxidizable serotonin (see Ref. 26 and references therein). To confirm the loading of serotonin into islet cells, the cytoplasmic accumulation was assessed indirectly using its fluorescent analog 5,7-dihydroxytryptamine (Supplemental Fig. 6A). All islet cells including cells smaller than 7 μm gradually became fluorescent. In addition, immunostaining with vesicular monoamine transporter (VMAT) antibodies revealed that both α- and β-cells expressed VMAT2 (Supplemental Fig. 6B) but not VMAT1 (data not shown). After the serotonin loading, fusion of individual vesicles was detected as spikes of oxidation current (Fig. 6A) (see Ref. 26). Upon application of kainate, the average rate of exocytosis, defined as the number of spikes per 30-sec time-bin, transiently increased within 30 sec in small islet cells (diameter, <7 μm, n = 17; Fig. 6B). These small cells did not respond to 16.7 mmol/liter glucose. As expected, a group of large cells (>11 μm), which should lack functional iGluRs (Fig. 1), secreted in response to 16.7 mmol/liter glucose but not to kainate (n = 7, Fig. 6C). Thus, we conclude that in α-cells, the activation of AMPA receptors can elicit exocytosis.
Discussion
Cell types expressing AMPAR in mouse islets
It is now well accepted that some pancreatic islet cells express functional glutamate receptors and that their activation modulates hormone secretion (4). Many studies have investigated the function of iGluRs by measuring secretion of islet hormones in intact islets or in perfused pancreas (6,15,16). Such data should be interpreted carefully, however, due to possible paracrine or autocrine interactions between the cells. For example, insulin secretion might be stimulated by glucagon (34) released by the activation of iGluRs in α-cells even if β-cells themselves lacked functional iGluRs. Therefore, Gromada et al. (5) suggested that tests of glutamate actions might have to be done in isolated single cells to avoid potentially confounding paracrine interactions. Some groups have reported l-glutamate-induced currents or membrane depolarization in single β-cells. The cells were identified either by [Ca2+]i rises in high glucose or by their relatively large size and the predominance of β-cells in islet-cell preparations. However, even though islet cells fall into two size classes, the intermediate range of cell sizes, where β- and non-β-cells overlap, may have obscured cell identification (Fig. 1B).
Our initial single-cell screens based on cell size suggested that functional iGluRs are found only in non-β-cells (Fig. 1). A similar conclusion was drawn in experiments in which we distinguished cell types based on intrinsic Ca2+ responses to glucose (Supplemental Fig. 7). Other laboratories have reported with cultured β-cells that [Ca2+]i oscillates in high glucose but not in low glucose (e.g. a cell shown in Supplemental Fig. 7A) (35). None of such cells responded to kainate (23 cells out of 79 tested cells). α-Cells are known to be unresponsive to high concentrations of glucose (e.g. a cell shown in Supplemental Fig. 7B) (36,37). However, we observed that some of the dispersed single α-cells, under culture conditions, strangely responded to high glucose level as well (e.g. a cell shown in Supplemental Fig. 7C) (38,39). Those presumed α-cells showed Ca2+ increases upon kainate application (36 cells for the cell groups as Supplemental Fig. 7B and 20 cells for the cell groups as Supplemental Fig. 7C). In spite of some uncertainty, the identification of islet cell types using the glucose response supports our hypothesis that non-β-cells but not β-cells express iGluRs. More definitive conclusions for the cell type expressing iGluRs came from our parallel [Ca2+]i measurement and hormone immunocytochemistry in single cells, indicating that α-cells, but not β- or δ-cells, express functional iGluRs in mouse islets (Fig. 2), and from measurement of the change of Cm in islet cells identified using single-cell RT-PCR (Fig. 3). This conclusion is in line with a recent study that was published during the preparation of this paper (16).
Our study and previous ones suggest that AMPA receptor immunoreactivity is present in β cells as well (10). We do not fully understand why iGluRs in β-cells show no function. Possibly iGluRs recycle between the pools in the plasma membrane and cytoplasm as at neuronal synapses (40), and most iGluRs of β-cells are in the cytoplasmic pool. This assertion is supported by the presence of cytoplasmic iGluR2 in our immunostaining (Supplemental Fig. 1). It would be interesting to address whether iGluRs in β-cells acquire functionality in certain circumstances such as elevated glucose concentration.
The subtype of iGluRs expressed in α-cells
We found that the iGluRs of pancreatic islets are AMPA receptors rather than kainate receptors on the basis of three criteria: their preferential activation and desensitization by AMPA, weak- and nondesensitizing activation by kainate, and the block of desensitization by cyclothiazide. The mRNA transcript for iGluR2 subunits in adult brains is frequently edited so that a glutamine codon initially present in the RNA is altered to an arginine codon (41). In neurons, channels with higher iGluR2 content in the edited form are characterized by a linear I-V relation and a low Ca2+ permeability, whereas channels lacking iGluR2 subunits show a doubly rectifying I-V relation and a high Ca2+ permeability (30,42). Therefore, islet AMPA receptors probably include edited iGluR2 subunits. Previous immunohistochemical studies concluded that AMPA receptors are composed mainly of iGluR2/3 in α- and β-cells but not δ-cells of rat intact islets (10). Our data using mouse islets are in line with those findings (Supplemental Fig. 1). The AMPA receptors expressed in islet cells desensitize rapidly and completely with l-glutamate. Such 5-msec desensitization is a functional signature of AMPA receptors in GABAergic interneurons (43,44) and distinct from the receptors on neocortical glutamatergic neurons, which have slower kinetics (τ = 10–16 msec) (33). In addition to the subunit composition, flip-flop splice variants are another determinant of receptor desensitization (45,46). Of them, the “flop” variants are relatively faster in desensitization (τ = 5–6 msec) with less than 1% nondesensitizing current, similar to what we saw for islet AMPA receptors (45).
Functions of AMPA receptors expressed in mouse islets
In CNS neurons, excitatory neurotransmitters are released from presynaptic terminals, diffuse 10–20 nm across the synaptic cleft, and activate postsynaptic receptors to evoke excitatory postsynaptic potentials (47). The time course of l-glutamate concentration in the synaptic cleft is determined by receptor binding and by clearance by glutamate transporters. In pancreatic islets, the release of l-glutamate and localization of iGluRs would be less well organized than in the synapse, so activation of the receptors might be less effective. Nevertheless, morphological studies reveal tight junctions and gap junctions between islet cells with narrow intercellular spaces of only 15–20 nm (48). Future studies on l-glutamate release and diffusion in the extracellular space will be critical to determine how rapidly desensitizing iGluRs elicit cellular reactions as we demonstrated in this study. In our mouse islet cells, the ultrafast application of l-glutamate rapidly depolarized the membrane of small cells by about 30 mV. This membrane depolarization lasted 12 times longer than the decay time constant of iGluR-mediated current measured under voltage-clamp mode and lasted much longer than the expected passive decay of small electrical signals for such cells. For example, our responsive cells had a Cm of 3–4 pF and an input resistance (Rm) of 1 GΩ at −70 mV, giving an expected passive electrical decay time constant (RmCm) of only 3–4 msec. Therefore, the depolarization must be secondarily prolonged by the induced activation of other depolarizing channels such as voltage-gated Ca2+ channels (Fig. 5). The final event of iGluR activation in α-cells is glucagon secretion as determined by our single cell methods and by glucagon released from islets (16,17).
The α-cells are regarded as the site of l-glutamate production in the pancreas because glutaminase activity is confined to the mantle of the islets (49). Thus glucagon secretion would be potentiated by a glutamatergic autocrine positive feedback after low glucose stimulation in α-cells. Because glucagon is known to activate β-cells via glucagon receptors (34), the glutamatergic signaling can further regulate insulin secretion in a limited time window. Therefore, our results define the iGluRs that fine-tune blood glucose levels and expand our understanding of l-glutamate as an intercellular signal in peripheral systems.
Supplementary Material
Acknowledgments
We thank Joseph G. Duman and Lindsey Burnett for helpful advice about immunocytochemistry and Arnold Sipos for confocal microscopy.
Footnotes
Present address for J.-H.C. and R.H.C.: Department of Physiology and Biophysics, Keck School of Medicine, Zilkha Neurogenetic Institute, University of Southern California, Los Angeles, California 90089.
This work was supported by the Korea Science and Engineering Foundation Grant R01-2002-000-00285-0 (to D.-S.K.), National Institutes of Health Grants GM83913 (to B.H.) and DK60623 and GM85791 (to R.H.C.), and by the Diabetes Endocrinology Research Center of the University of Washington.
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 26, 2010
Abbreviations: AMPA, α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate; Cm, membrane capacitance; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; CNS, central nervous system; iGluR, ionotropic glutamate receptor; I-V, current-voltage; NMDG, N-methyl-D-glucamine; VMAT, vesicular monoamine transporter.
References
- Wisden W, Seeburg PH 1993 Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol 3:291–298 [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S 1994 Cloned glutamate receptors. Annu Rev Neurosci 17:31–108 [DOI] [PubMed] [Google Scholar]
- Hinoi E, Takarada T, Ueshima T, Tsuchihashi Y, Yoneda Y 2004 Glutamate signaling in peripheral tissues. Eur J Biochem 271:1–13 [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Hayashi M 2003 Glutamate-mediated signaling in the islets of Langerhans: a thread entangled. Trends Pharmacol Sci 24:511–517 [DOI] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB 2007 α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28:84–116 [DOI] [PubMed] [Google Scholar]
- Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, Rorsman P, Braun M 2004 Glucose inhibition of glucagon secretion from rat α-cells is mediated by GABA released from neighboring β-cells. Diabetes 53:1038–1045 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Yamada H, Uehara S, Morimoto R, Muroyama A, Yatsushiro S, Takeda J, Yamamoto A, Moriyama Y 2003 Secretory granule-mediated co-secretion of L-glutamate and glucagon triggers glutamatergic signal transmission in islets of Langerhans. J Biol Chem 278:1966–1974 [DOI] [PubMed] [Google Scholar]
- Bai L, Zhang X, Ghishan FK 2003 Characterization of vesicular glutamate transporter in pancreatic α- and β-cells and its regulation by glucose. Am J Physiol Gastrointest Liver Physiol 284:G808–G814 [DOI] [PubMed] [Google Scholar]
- Weaver CD, Gundersen V, Verdoorn TA 1998 A high affinity glutamate/aspartate transport system in pancreatic islets of Langerhans modulates glucose-stimulated insulin secretion. J Biol Chem 273:1647–1653 [DOI] [PubMed] [Google Scholar]
- Weaver CD, Yao TL, Powers AC, Verdoorn TA 1996 Differential expression of glutamate receptor subtypes in rat pancreatic islets. J Biol Chem 271:12977–12984 [DOI] [PubMed] [Google Scholar]
- Weaver CD, Partridge JG, Yao TL, Moates JM, Magnuson MA, Verdoorn TA 1998 Activation of glycine and glutamate receptors increases intracellular calcium in cells derived from the endocrine pancreas. Mol Pharmacol 54:639–646 [PubMed] [Google Scholar]
- Gonoi T, Mizuno N, Inagaki N, Kuromi H, Seino Y, Miyazaki J, Seino S 1994 Functional neuronal ionotropic glutamate receptors are expressed in the non-neuronal cell line MIN6. J Biol Chem 269:16989–16992 [PubMed] [Google Scholar]
- Inagaki N, Kuromi H, Gonoi T, Okamoto Y, Ishida H, Seino Y, Kaneko T, Iwanaga T, Seino S 1995 Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J 9:686–691 [PubMed] [Google Scholar]
- Molnár E, Váradi A, McIlhinney RA, Ashcroft SJ 1995 Identification of functional ionotropic glutamate receptor proteins in pancreatic β-cells and in islets of Langerhans. FEBS Lett 371:253–257 [DOI] [PubMed] [Google Scholar]
- Bertrand G, Gross R, Puech R, Loubatières-Mariani MM, Bockaert J 1992 Evidence for a glutamate receptor of the AMPA subtype which mediates insulin release from rat perfused pancreas. Br J Pharmacol 106:354–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera O, Jacques-Silva MC, Speier S, Yang SN, Köhler M, Fachado A, Vieira E, Zierath JR, Kibbey R, Berman DM, Kenyon NS, Ricordi C, Caicedo A, Berggren PO 2008 Glutamate is a positive autocrine signal for glucagon release. Cell Metabolism 7:545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand G, Gross R, Puech R, Loubatières-Mariani MM, Bockaert J 1993 Glutamate stimulates glucagon secretion via an excitatory amino acid receptor of the AMPA subtype in rat pancreas. Eur J Pharmacol 237:45–50 [DOI] [PubMed] [Google Scholar]
- Muroyama A, Uehara S, Yatsushiro S, Echigo N, Morimoto R, Morita M, Hayashi M, Yamamoto A, Koh DS, Moriyama Y 2004 A novel variant of ionotropic glutamate receptor regulates somatostatin secretion from δ-cells of islets of Langerhans. Diabetes 53:1743–1753 [DOI] [PubMed] [Google Scholar]
- Tong Q, Ouedraogo R, Kirchgessner AL 2002 Localization and function of group III metabotropic glutamate receptors in rat pancreatic islets. Am J Physiol Endocrinol Metab 282:E1324–E1333 [DOI] [PubMed] [Google Scholar]
- Uehara S, Muroyama A, Echigo N, Morimoto R, Otsuka M, Yatsushiro S, Moriyama Y 2004 Metabotropic glutamate receptor type 4 is involved in autoinhibitory cascade for glucagon secretion by α-cells of islet of Langerhans. Diabetes 53:998–1006 [DOI] [PubMed] [Google Scholar]
- Chen L, Koh DS, Hille B 2003 Dynamics of calcium clearance in mouse pancreatic β-cells. Diabetes 52:1723–1731 [DOI] [PubMed] [Google Scholar]
- Vignali S, Leiss V, Karl R, Hofmann F, Welling A 2006 Characterization of voltage-dependent sodium and calcium channels in mouse pancreatic A- and B-cells. J Physiol 572:691–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ 1981 Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100 [DOI] [PubMed] [Google Scholar]
- Koh DS, Hille B 1997 Modulation by neurotransmitters of catecholamine secretion from sympathetic ganglion neurons detected by amperometry. Proc Natl Acad Sci USA 94:1506–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY 1985 A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450 [PubMed] [Google Scholar]
- Zhou Z, Misler S 1996 Amperometric detection of quantal secretion from patch-clamped rat pancreatic β-cells. J Biol Chem 271:270–277 [DOI] [PubMed] [Google Scholar]
- Koh DS, Hille B 1999 Rapid fabrication of plastic-insulated carbon-fiber electrodes for micro-amperometry. J Neurosci Methods 88:83–91 [DOI] [PubMed] [Google Scholar]
- Lewis CA 1979 Ion-concentration dependence of the reversal potential and the single channel conductance of ion channels at the frog neuromuscular junction. J Physiol 286:417–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipeleers DG, Pipeleers-Marichal MA 1981 Method for the purification of single A, B and D cells and for the isolation of coupled cells from isolated rat islets. Diabetologia 20:654–663 [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF 1999 The glutamate receptor ion channels. Pharmacol Rev 51:7–61 [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A 1993 Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron 11:1069–1082 [DOI] [PubMed] [Google Scholar]
- Iino M, Ozawa S, Tsuzuki K 1990 Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol 424:151–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Sakmann B 1992 Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol 455:143–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer TJ, Heller RS, Unson CG, Weir GC, Habener JF 1996 Distribution of glucagon receptors on hormone-specific endocrine cells of rat pancreatic islets. Endocrinology 137:5119–5125 [DOI] [PubMed] [Google Scholar]
- Henquin JC, Jonas JC, Gilon P 1998 Functional significance of Ca2+ oscillations in pancreatic β cells. Diabetes Metab 24:30–36 [PubMed] [Google Scholar]
- Berts A, Ball A, Gylfe E, Hellman B 1996 Suppression of Ca2+ oscillations in glucagon-producing α 2-cells by insulin/glucose and amino acids. Biochim Biophys Acta 1310:212–216 [DOI] [PubMed] [Google Scholar]
- Berts A, Gylfe E, Hellman B 1995 Ca2+ oscillations in pancreatic islet cells secreting glucagon and somatostatin. Biochem Biophys Res Commun 208:644–649 [DOI] [PubMed] [Google Scholar]
- Salehi A, Vieira E, Gylfe E 2006 Paradoxical stimulation of glucagon secretion by high glucose concentrations. Diabetes 55:2318–2323 [DOI] [PubMed] [Google Scholar]
- Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J 2005 Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in β-cells. Endocrinology 146:4861–4870 [DOI] [PubMed] [Google Scholar]
- Passafaro M, Piëch V, Sheng M 2001 Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci 4:917–926 [DOI] [PubMed] [Google Scholar]
- Sommer B, Köhler M, Sprengel R, Seeburg PH 1991 RNA editing in brain controls a determinant of ion flow in glutamate-gated channels. Cell 67:11–19 [DOI] [PubMed] [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H 1995 Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15:193–204 [DOI] [PubMed] [Google Scholar]
- Livsey CT, Costa E, Vicini S 1993 Glutamate-activated currents in outside-out patches from spiny versus aspiny hilar neurons of rat hippocampal slices. J Neurosci 13:5324–5333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh DS, Geiger JR, Jonas P, Sakmann B 1995 Ca2+ permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol 485:383–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Keinänen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Köhler M, Takagi T, Sakmann B, Seeburg PH 1990 Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science 249:1580–1585 [DOI] [PubMed] [Google Scholar]
- Mosbacher J, Schoepfer R, Monyer H, Burnashev N, Seeburg PH, Ruppersberg JP 1994 A molecular determinant for submillisecond desensitization in glutamate receptors. Science 266:1059–1062 [DOI] [PubMed] [Google Scholar]
- Clements JD 1996 Transmitter timecourse in the synaptic cleft its role in central synaptic function. Trends Neurosci 19:163–171 [DOI] [PubMed] [Google Scholar]
- Orci L, Malaisse-Lagae F, Ravazzola M, Rouiller D, Renold AE, Perrelet A, Unger R 1975 A morphological basis for intercellular communication between α- and β-cells in the endocrine pancreas. J Clin Invest 56:1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero F, Baglietto-Vargas D, Moreno-González I, López-Tellez JF, Cuesta-Munoz AL, Gutiérrez A, Aledo JC 2007 Glutaminase activity is confined to the mantle of the islets of Langerhans. Biochimie 89:1366–1371 [DOI] [PubMed] [Google Scholar]
- Renström E, Eliasson L, Bokvist K, Rorsman P 1996 Cooling inhibits exocytosis in single mouse pancreatic B-cells by suppression of granule mobilization. J Physiol 494(Pt 1):41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.