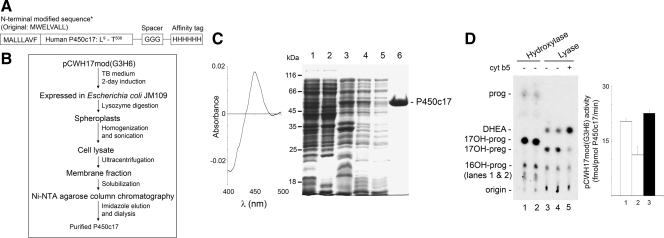
Figure 1.
Construction, expression, and purification of recombinant human P450c17. A, Diagram of the modified form of human P450c17 expressed by pCWH17mod(G3H6); single letter amino acid codes are used to indicate the relevant sequences. The eight N-terminal residues are modified as described (11), the residues from Leu9 to Thr508 are those of wild-type P450c17 (4,5), and the Gly3His6 tag precedes the stop codon. B, Flow chart of purification of recombinant human P450c17 from bacteria. C, Purification of recombinant human P450c17 from bacteria. Dithionite-reduced carbon monoxide-difference spectra of pCWH17mod(G3H6) is shown in the left panel. The specific content is 9.9 pmol/μg protein. In the right panel, samples from various purification steps were analyzed by 12% SDS-PAGE and stained with Coomassie brilliant blue. Lane 1, Cell lysate (∼50 μg protein); lane 2, supernatant from ultracentrifugation (∼50 μg); lane 3, membrane fraction (pellet from ultracentrifugation) (∼25 μg); lane 4, solubilized membrane fraction (∼10 μg); lane 5, flow-through from the Ni-NTA agarose column (∼10 μg); lane 6, purified recombinant human P450c17 (10 μg). The migration of molecular weight markers (kilodaltons) is shown on the left. D, 17α-Hydroxylase and 17,20 lyase activities of the bacterially expressed recombinant human P450c17. Left panel, Radiolabeled progesterone (prog) was used as the substrate for the hydroxylase assays (lanes 1 and 2), and 17OH-preg was used as the substrate for the lyase assays (both at 1 μm). The hydroxylase assay yields 17OHP (17OH-prog) and a small amount of 16OH-progesterone (16OH-prog); the lyase reaction yields dehydroepiandrosterone (DHEA). Some minor contaminant in the substrate steroids are not metabolized. The assays were done in duplicates in lanes 1 and 2 for the hydroxylase reaction and lanes 3 and 4 for the lyase reaction. In lane 5, 10 pmol cytochrome b5 were added to the lyase reaction. Right panel, The activities of pCWH17mod(G3H6) were quantitated for the hydroxylase assay (lane 1), lyase assay in the absence of cytochrome b5 (lane 2), and 17,20 lyase activity in the presence of 10 pmol cytochrome b5 (lane 3); data are mean ± sd.