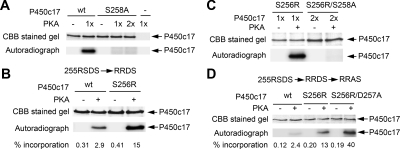
Figure 2.
PKA phosphorylates Ser258. In each panel, the amount of P450c17 is indicated by staining with Coomassie brilliant blue (CBB), and phosphorylation is assessed by autoradiography of incorporated 32P. A, S258A is not phosphorylated. Wild-type (wt) and S258A human P450c17 (3 μg each) were treated without (−) and with (+) 0.5 U PKA catalytic subunit (one time) or with 1.0 U PKA catalytic subunit (two times) and 20 μm ATP (1μCi γ-32P-ATP) for 30 min at 30 C. B, S256R increases phosphorylation. S256R and wild-type (wt) P450c17 (5 μg each) were incubated with 0.5U PKA catalytic subunit for 10 min at 30 C. The percentages of 32P incorporated (picomoles 32P per picomole P450c17) of each sample are indicated below the figure. C, S256R does not promote phosphorylation of S258A. S256R mutant (1 μg) or S256R/S258A double mutant (2 μg) was treated with 0.5 U PKA catalytic subunit for 30 min at 30 C. D, D257A promotes phosphorylation. Wild-type human P450c17, S256R mutant, and S256R/D257A double mutant (0.75 μg each) were treated with 0.5 U PKA catalytic subunit for 10 min at 30 C. The percentages of 32P incorporation are indicated below the figure, as in B.