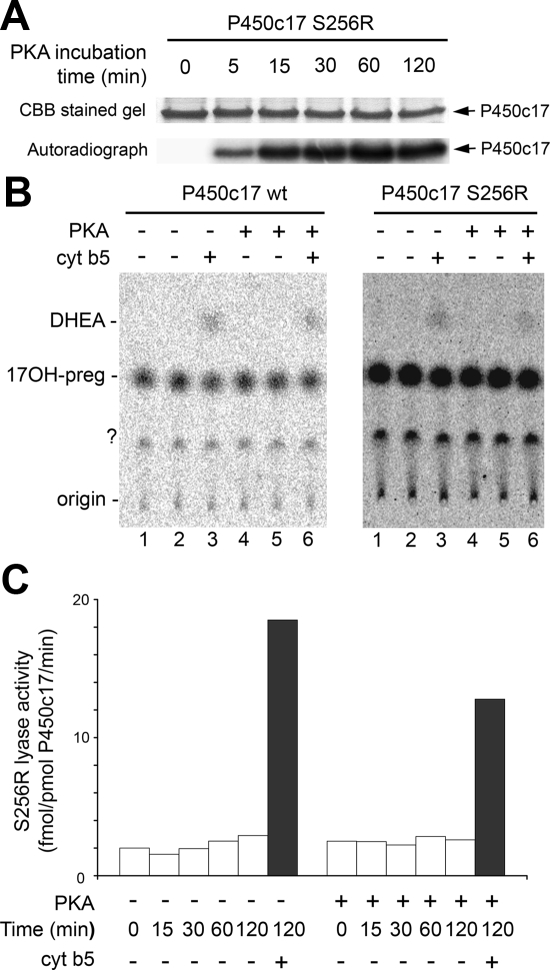
Figure 3.
Effect of S258 phosphorylation on catalysis by P450c17. A, The S256R mutant (4 μg) was phosphorylated by 0.5 U PKA catalytic subunit with 20 μm ATP (1 μCi γ-32P-ATP) for the indicated times and analyzed by SDS-PAGE. Staining with Coomassie brilliant blue (CBB) and autoradiography of incorporated 32P are shown. B, Unphosphorylated and PKA-phosphorylated P450c17 wild-type (left panel) and S256R mutant (right panel) (10 pmol each) were assayed for 17,20 lyase activity with 1 μm 3H-17OH-preg. The lane numbers identify corresponding experiments in each panel. Each form of P450c17 was preincubated in the presence or absence of PKA for 30 min at 30 C followed by lyase assay for 2 h in the absence or presence of 10 pmol cytochrome b5 (cyt b5) in lanes 3 and 6. The experiment was done in duplicates for lanes 1–2 and 4–5 for the unphosphorylated and PKA-phosphorylated forms of P450c17, respectively. DHEA, Dehydroepiandrosterone. C, The S256R mutant was preincubated in the absence or presence of PKA for the indicated times followed by 2 h incubation with steroids for the 17,20 lyase assay in the absence (open bars) or presence (closed bars) of cytochrome b5. The data are means from duplicate experiments.