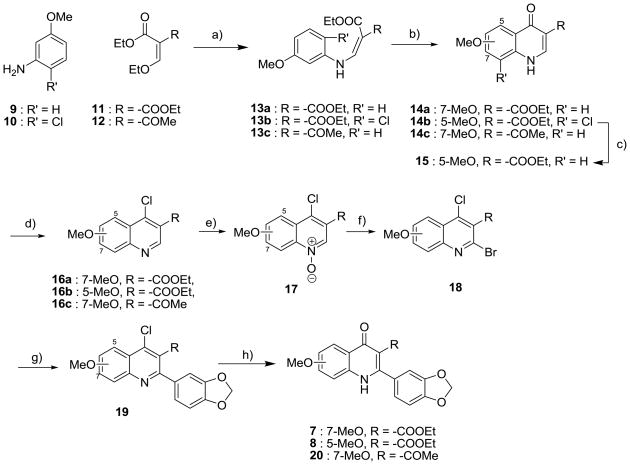
Scheme 1. Synthesis of 4(1H)-quinolones.
a Reagents and conditions: a) EtOH, 130 °C, 3 h; b) Ph2O, reflux, 4–6 h; c) 5% Pd/C, H2, MeOH, rt, overnight; d) POCl3, 1,4-dioxane, 120 °C, 1 h; e) m-chloroperbenzoic acid, CHCl3, rt, 4 h; f) POBr3, CHCl3, rt, 1 h. g) 3,4-methylenedioxyphenyl boronic acid, Pd(PPh3)4, CsCO3, 1,4-dioxane/H2O, 75 °C, 3 h; h) AcOH/H2O (4:1), 120 °C, 1 h.