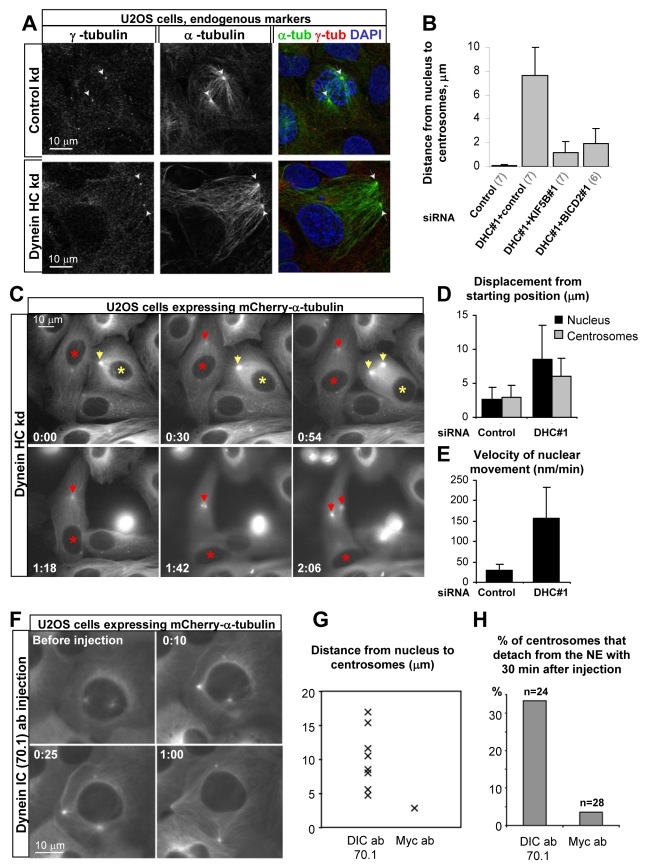
Figure 5. Dynein inactivation causes rapid separation of nuclei and centrosomes in G2.
(A) U2OS cells were transfected with the control or DHC#3 siRNAs, fixed with cold methanol, and stained for α-tubulin (green in overlay) and γ-tubulin (red in overlay), as well as DNA (DAPI, blue in overlay). Note that centrosomes are located close to the nucleus in control cells but are removed far away from the nucleus in dynein-depleted cells. (B) mCherry-α-tubulin stable U2OS cell line was imaged with a 3 min time interval 2.5 d after transfection with the indicated siRNA mixtures, and the distance between centrosomes and the nucleus at the time of NEB was measured. Error bars represent SD. The number of experiments is indicated in parentheses below the graph. ∼20 cells per experiment were analyzed. (C) mCherry-α-tubulin stable U2OS cells were imaged with a 3 min interval 2.5 d after transfection with DHC#1 siRNA. Nuclei and centrosomes undergo G2-specific displacements (indicated by asterisks and arrows, respectively), resulting in their separation. (D,E) Quantification of displacement of nuclei and centrosomes starting from the moment when G2-specific enhanced nucleation of MTs at the centrosome became visible (D), and velocity of nuclear movement during 1 h before NEB (E). Nuclear movement in dynein-depleted cells was initiated 54±4 min (mean±SD) before NEB. Measurements were performed in 10 cells in 3 independent experiments. (F–H) U2OS cells stably expressing mCherry-α-tubulin were microinjected with antibodies against dynein IC (70.1) or the Myc tag. Late G2 cells were chosen based on the presence of separated centrosomes. (F) An example of nucleus-centrosome separation after microinjection with dynein-inhibiting antibodies. (G,H) Distance between the nucleus and the centrosomes (G) and percentage of cells showing strong centrosome detachment (H) at 30 min after microinjection.