Figure 6.
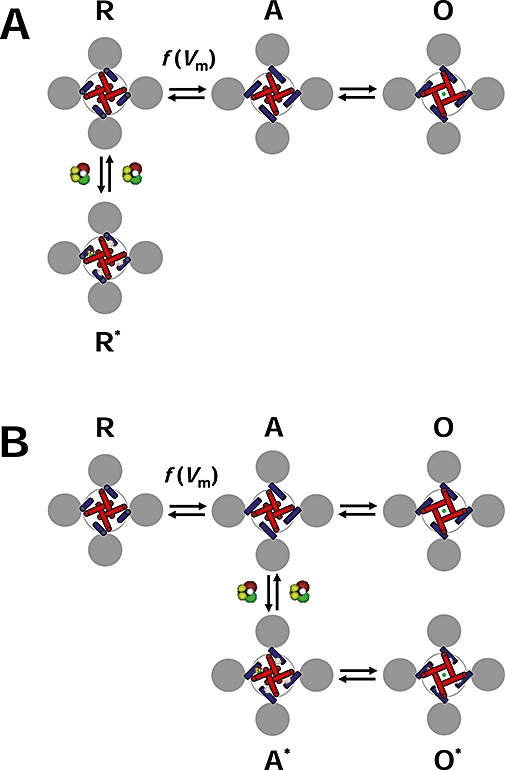
Diagram of the allosteric modulation of Shaw2 channels by halothane. (A) Working hypothesis of the inhibition of the Shaw2 channel by halothane. The drawing represents the tetrameric Shaw2 channel viewed from cytoplasmic side. The grey circles represent the voltage sensing domains (VSDs), the blue cylinders represent the S4S5 linkers that connect the VSDs to the pore domain (PD) and the red cylinders represent the S6 helix bundle that forms the activation gate of the K+ channel in the PD. The symbols R, A and O label three distinct conformations of the channel: resting-closed, pre-open activated and open respectively. Note that the voltage-dependent movement of the VSDs displaces the S4S5 linkers to activate the channel; and consequently, the S6 helix bundle undergoes a concerted conformational change to open the channel. The green circle represents K+ in the pore. Whereas channel activation is governed by voltage-dependent rate constants [f(Vm)], the final concerted opening step may only depend weakly on the membrane potential. To explain the inhibition of the Shaw2 channel by halothane, we hypothesize that it binds to the resting closed conformation R preferentially and stabilizes it (R*). The interfaces between the S4S5 linker and the distal S6 segment are the putative binding sites. Binding to one site is sufficient to stabilize the resting closed conformation. (B) Working hypothesis of the potentiation of the Shaw2 P410A channel by halothane. This diagram uses the same conventions as described above. When the P410A mutation in the PVPV motif reduces the kink in distal segment of S6, halothane may bind to the pre-open activated conformation (A), and thereby stabilizes it (A*). Consequently, channel opening (O*) is more likely than channel deactivation, and the current is potentiated. Although the local structural change induced by the P410A mutation may have changed the relationship between the S4S5 linker and distal S6, the interface between these regions may still constitute the binding site for halothane.
