Table IV.
Pharmacokinetic Parameters for SMEDDS B and Capsule Suspension
| Parameters | SMEDDS B | Capsule suspension |
|---|---|---|
| t max (h) | 1 ± 0.44 | 1.36 ± 0.39 |
| C max (ng/mL) | 112.61 ± 9.13 | 69.24 ± 3.99 |
| AUC0→t (ng h/mL) | 607.93 ± 45.06 | 445.36 ± 70.50 |
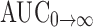 (ng h/mL) (ng h/mL) |
1,124.57 ± 79.66 | 893.72 ± 116.56 |
| AUMC0→t (ng h/mL) | 4,752.96 ± 102.70 | 3,848.13 ± 265.20 |
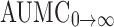 (ng h/mL) (ng h/mL) |
37,933.75 ± 1,609.08 | 33,804.48 ± 1,761.19 |
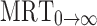 (h) (h) |
33.73 ± 1.11 | 37.82 ± 1.40 |
| Relative bioavailability (%) | 178.70 | – |
SMEDDS self-microemulsifying drug delivery system, t
max time of peak concentration, C
max peak of maximum concentration, AUC
0→t area under the concentration time profile curve until last observation, 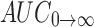 area under the concentration time profile curve extrapolated to infinity, AUMC
0→t area under moment curve computed to the last observation,
area under the concentration time profile curve extrapolated to infinity, AUMC
0→t area under moment curve computed to the last observation, 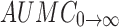 area under moment curve extrapolated to infinity,
area under moment curve extrapolated to infinity, 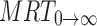 mean residence time
mean residence time
