Table I.
Pharmacokinetic Parameters of Celecoxib in Human Volunteers (n = 6) After Administration of 200 mg as Single Oral Dose of Proniosomal Formulation and Celebrex® Capsules
| PK parameter | Conventional capsule | Proniosomal capsule |
|---|---|---|
| C max (ng/ml) | 705.17 ± 31.89 | 842.5 ± 34.72* |
| AUC0–48 (ng.h/ml) | 6,761.58 ± 612.73 | 11,681.21 ± 523.90* |
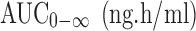
|
7,210.58 ± 689.02 | 12,406.21 ± 469.11*a |
| T max (h) | 2.5 ± 0.55 | 4 ± 0.00** |
| K a (h−1) | 1.52 ± 0.17 | 0.73 ± 0.05* |
| K el (h−1) | 0.051 ± 0.01 | 0.059 ± 0.002 |
| t 1/2 (h) | 13.33 ± 1.98 | 11.75 ± 0.55 |
Each value represents the mean ± standard division of six subjects
*P < 0.05 based on ANOVA, statistically significant difference between conventional and proniosomal capsules
**P < 0.05 based on Wilcoxon signed rank test, statistical significant difference between conventional and proniosomal capsules
