Abstract
In the present investigation, hydrogenated cottonseed oil (HCSO) was evaluated as a sustained release matrix for a freely soluble drug, tramadol. Hydrophobic matrix tablets of tramadol, was evaluated by compression of physical mixture of drug and wax, dispersion of drug in HCSO by hot fusion or solubilisation techniques. The method of preparation of tablet had a significant effect on drug release with higher release observed from direct compression matrices and slower release from matrix prepared by dispersion (hot-fused matrices). Influence of addition of hydroxypropylmethyl cellulose, sodium carboxymethyl cellulose, polyethylene glycol 4000 and surfactants like sodium lauryl sulphate and polysorbate 20 to HCSO matrix on drug release was investigated. The added excipients exhibited a propensity to enhance drug release from the HCSO matrix. NaCMC was effective at a lower ratio (<10% w/w) and when incorporated at higher level made HCSO matrix to erode and disintegrate in a short period.
Key words: Diffusion, Hydrogenated cottonseed oil, Matrix tablet, Sustained release, Tramadol
INTRODUCTION
Drug solubility is an important factor while designing a sustained release formulation since freely water soluble drugs always poses a great challenge to the formulator. Under these circumstances, erosion or swelling matrix or coating measures can be applied as a mean to retard the drug release (1). But erosion-controlled or swelling-controlled systems often are poor barriers to provide the desired release profile for freely water soluble drugs. Since a freely soluble drug diffuses easily through the hydrophilic gel network, a hydrophobic polymer or wax matrix is highly preferred. Their utility over hydrophilic polymer is advantageous as they give good stability to the product at varying pH level (2).
Wax or lipids have been frequently tested for controlling the release of freely water soluble drugs from the matrix (3). These materials when incorporated in sufficient amount result in formation of a matrix system and provide sustained release of drug (4). The impact of wax type on drug release is worth investigating (5–8). In the past, a variety of waxes like Compritol® (5–7), Precirol® (9,10), Compactrol® (3), bees wax and stearic acid (11) have been investigated with pharmaceutical applications.
Hydrogenated cottonseed oil (HCSO), widely used as a tablet lubricant, was investigated in the present study as a hydrophobic matrix forming material. It belongs to USPNF type 1 HCSO consisting of triglycerides of hydroxy stearic acid. It melts and re-solidifies during compaction process enhancing the bonding capacity of the tablet matrix (12). But the application of HCSO as a matrix material in controlling drug release has not been studied in detail (2,3,12) and hence it is worthwhile to evaluate HCSO as a matrix material.
Tramadol HCl, used in present study as a model drug, belongs to opioid analgesic used to relieve moderate to severe pain without any serious side effects. Its usual oral dosage regimen is 50-100 mg every 4-6 h up to 400 mg/day and has a half-life of ∼5.5 h (14). On oral administration, it is readily absorbed and is predominately excreted as a metabolite in the urine. For the compliance of patients, therefore, it is desirable to formulate tramadol into sustained release formulation (15).
The present investigation was designed with a hypothesis that HCSO being a wax has a propensity to prolong freely water soluble drugs. Tramadol HCl (water solubility: 30-100 mg/ml) was used as a model drug. To investigate the influence of drug solubility on drug release, besides tramadol, theophylline, a sparingly water-soluble drug (∼8.3 mg/ml) was used as a model drug.
MATERIALS AND METHODS
Materials
Tramadol hydrochloride (mol. wt. 299.8; M.P 180-181°C) and HCSO (M.P 57-70°C; saponification value 175-200) were a generous gift of Lupin Ltd, Pune, India. Theophylline (anhydrous) was obtained from French Pharma, Chandigarh, India. Polyethylene glycol 4000 (PEG 4000, Avg. mol. wt. 3,500-4,500) and sodium lauryl sulphate (SLS) were purchased from Sd-fine chemicals, Mumbai, India. HPMC K4M was a generous gift of Colorcon Asia Pvt. Ltd, Goa, India and sodium carboxy methyl cellulose was procured from Loba chemie Pvt. Ltd, Mumbai, India. Tween 20 was procured from Himedia laboratory, Mumbai, India. All other chemicals were of analytical grade and commercially purchased.
Methods
Preparation of Matrix Tablets by Direct Compression
Drug, HCSO and all other excipients were passed through sieve # 80 before further processing. Weighed quantities of drug, HCSO and other excipients (Tables I and II) were thoroughly mixed in a poly bag for 10 min to get a uniform blend of the ingredients. The physical mixture of drug with added excipients was directly compressed on a single station tableting machine (Cadmach Engg, Ahmedabad, India), using a flat-faced 12.60-mm punches.
Table I.
Formulation of Different Batches of Matrix Tablets
| Ingredients | Amount (mg/tablet) | |||||
|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | |
| Tramadol | 50 | 100 | 150 | 100 | 100 | - |
| Theophylline anhydrous | - | - | - | - | - | 100 |
| Hydrogenated cotton seed oil | 188 | 188 | 188 | 138 | 238 | 188 |
All batches contained 2% w/w of talc and magnesium stearate
Table II.
Tablet Formulations with Water-Soluble, Swellable Polymers and Surfactants
| Ingredients | Amount (mg/tablet) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F7 | F8 | F9 | F10 | F11 | F12 | F13 | F14 | F15 | F16 | F17 | F18 | |
| Tramadol | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Hydrogenated cotton seed oil | 138 | 138 | 138 | 138 | 138 | 138 | 138 | 138 | 138 | 138 | 138 | 138 |
| HPMCa | 10 | 20 | - | - | - | - | - | - | - | - | - | - |
| NaCMCa | - | - | 5 | 10 | - | - | - | - | - | - | - | - |
| PEG 4000a | - | - | - | - | 10 | 20 | - | - | - | - | - | - |
| Lactosea | - | - | - | - | - | - | 10 | 20 | - | - | - | - |
| SLSa | - | - | - | - | - | - | - | - | 5 | 10 | - | - |
| Tween 20a | - | - | - | - | - | - | - | - | - | - | 5 | 10 |
aQuantity (% w/w) with respect of hydrogenated cotton seed oil
All batches contained 2% w/w of talc and magnesium stearate
Preparation of Matrix Tablets by Hot Fusion
Tablets were prepared by hot-fusion method (1,16), except and otherwise specified. HCSO was melted at 70°C and drug was dispersed in the molten wax under mild agitation to get a uniform dispersion using a four bladed mixer (Remi laboratories, Mumbai, India). The drug-wax dispersion was allowed to cool to room temperature, resulting solidified mass was pulverised and passed through sieve (# 22) to get granules. The granules, admixed with lubricant and glidant, were compressed on a single station tableting machine (Cadmach Engg, Ahmedabad, India), using a flat-faced 12.60-mm punches.
To investigate the effect of method of preparation on drug release, two batches of tablets consisting of 100 mg tramadol (F2) was prepared by direct compression of the physical mixture and wet granulation of physical mixture of drug, wax and other excipients using PVP K-30 in isopropyl alcohol (3% w/v).
Thermal Treatment of Tablets
Formulation (batch F2) prepared by direct compression (containing tramadol) were subjected to thermal sintering by placing tablets in an oven (Consolidated Electric Industries, Bangalore, India) in which temperature was maintained at 57°C for 1 h. At the end of respective time of thermal exposure tablets were removed, allowed to cool to room temperature. Evaluations of thermally sintered tablets were carried out at least after 24 h of thermal treatment.
Evaluation of Tablets
Drug Content
Drug content was determined by crushing tablets (n = 10) in a glass mortar and pestle, and extracting the drug in distilled water with continuous shaking on a rotatory shaker (Remi Instruments Ltd, Mumbai, India) for 24 h. The drug content in each extracted fluid was assayed using an ultraviolet (UV) spectrophotometer (UV- 1601, Shimadzu, Japan) at 272 nm against a suitable blank.
Hardness of Tablets
Tablet was placed between the holding anvil and the piston connected to the direct force reading gauge of tester (Pfizer tester). Force was applied to the anvil and the crushing strength that just causes the tablet to break was recorded.
Friability of Tablets
Preweighed tablets (n = 10) was placed in Roche friabilator (Riche-Rich Pharma, Bangalore, India), which was then operated for 100 revolutions (25 rpm, 4 min). The tablets were then dusted and reweighed to check lose in its weight. The friability was calculated using the equation:
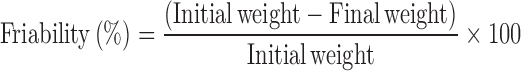 |
Weight Variation of Tablets
The average weight of 20 tablets was determined by individually weighing the tablets. By comparing the individual weights to the average weight, tablet weight variation was determined.
In vitro Drug Release
The drug release from the prepared tablets were studied using six station USP-23 dissolution rate test apparatus (Tab machines, Mumbai, India) with a paddle speed of 50 rpm. Dissolution medium consisted of 900 ml of degassed demineralised water maintained at 37 ± 0.2°C. At pre-identified time intervals, an aliquot was withdrawn and replenished with fresh medium. Amount of drug in each aliquot was assayed on a UV spectrophotometer (UV 1601, Shimadzu, Japan) at 272 nm against an appropriate blank. All trials were conducted in triplicate and the average (±SD) reading was noted.
Release Profile Analysis
The data obtained from the dissolution studies were fitted to zero-order equation (Eq. 1), first-order equation (Eq. 2) and mathematical equation (Eq. 3) described by Higuchi (17) to elucidate mechanism of drug release.
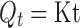 |
1 |
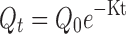 |
2 |
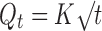 |
3 |
Where, Q is amount of drug released at time t, Q0 is the initial amount of drug in the dissolution medium, K is release constant. In order to further determine the mechanism of drug release data were fit to Korsmeyer-Peppas empirical power law equation (Eq. 4; 18) and Weibull distribution equation (Eq. 5; 19):
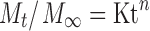 |
4 |
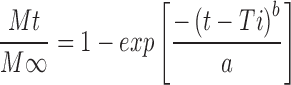 |
5 |
Where, Mt/M∞ is the fraction of drug released at time ‘t’, ‘K’ is the structural and geometrical constant, ‘n’ is the release exponent, ‘a’ defines scale parameter, ‘Ti’ represents lag time of dissolution process and ‘b ’ characterises shape parameter.
Statistical Analysis
The data obtained from the dissolution studies were statistically analysed by one-way ANOVA followed by Tukey method (SigmaStat v 3.0, SPSS, USA). A probability value of p < 0.05 was considered as statistically significant.
RESULTS
Preparation and Characterization of the Tablets
Drug content in all the formulations was in a range of 96.42 ± 0.75%-101.32 ± 0.87% exhibiting uniformity in drug. Weight of the prepared tablets was uniform consistently throughout the prepared batches. Friability and hardness of the prepared tablets was, respectively, <1% and 4.5-6.5 kg/cm2.
In Vitro Drug Release Studies
Effect of Drug Load and Processing Method on Release
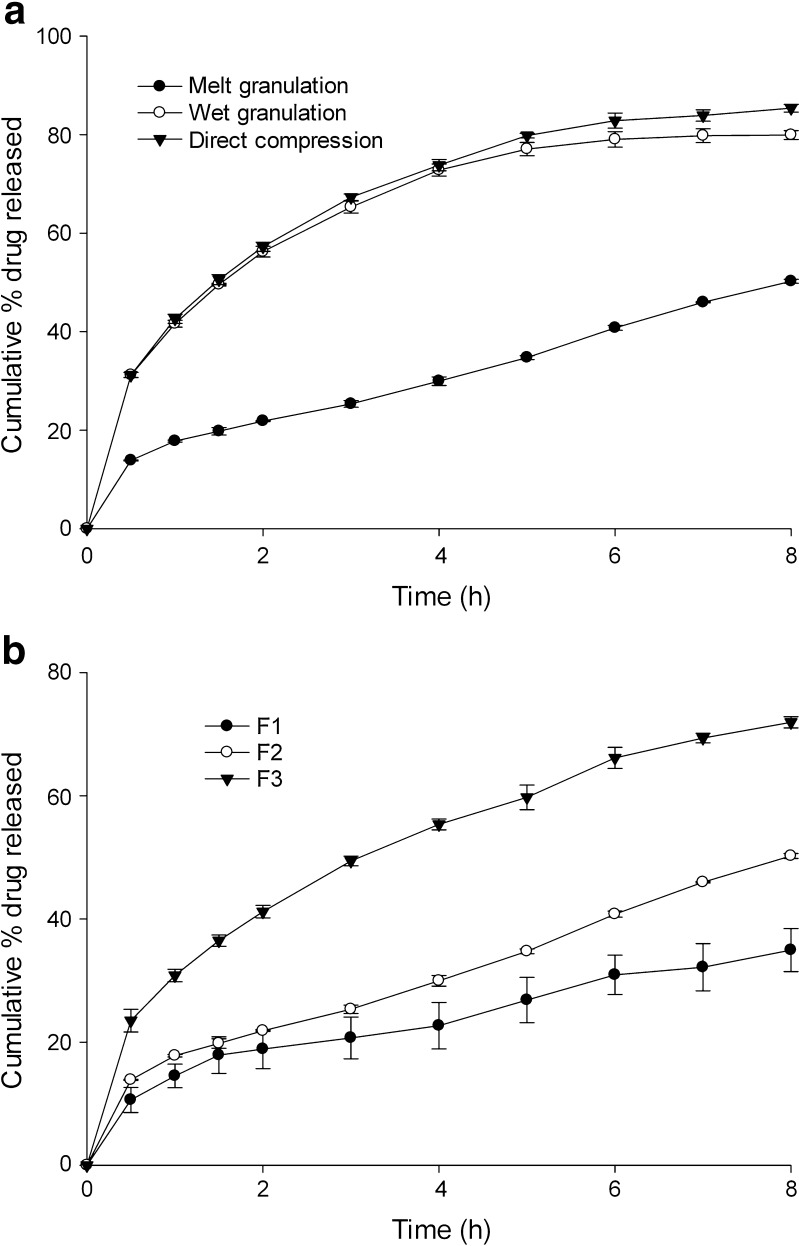
The processing method of tablets significantly altered tramadol release (Fig. 1). At constant wax and drug concentration, each matrix formation method resulted in different drug release profile. At the end of 8 h, 50.23 ± 0.73%, 79.90 ± 0.70% and 85.34 ± 0.34% of drug was depleted from formulation F2, respectively, from matrix prepared by hot fusion, wet granulation and direct compression.
Fig. 1.
Drug release profile influenced by a processing method and b drug concentration
Figure 1 shows the release profiles from tablets prepared by hot fusion containing 50, 100 or 150 mg tramadol keeping the wax level constant at 188 mg. The drug release from matrix tablets increased significantly (p < 0.05) with increase in tramadol concentration in matrix. About 34.25 ± 2.98%, 50.23 ± 0.73% and 71.92 ± 0.92% of drug was depleted from the matrix at the end of 8 h of dissolution study, respectively, from matrix having 50, 100 and 150 mg of drug.
Effect of Wax Level on Release
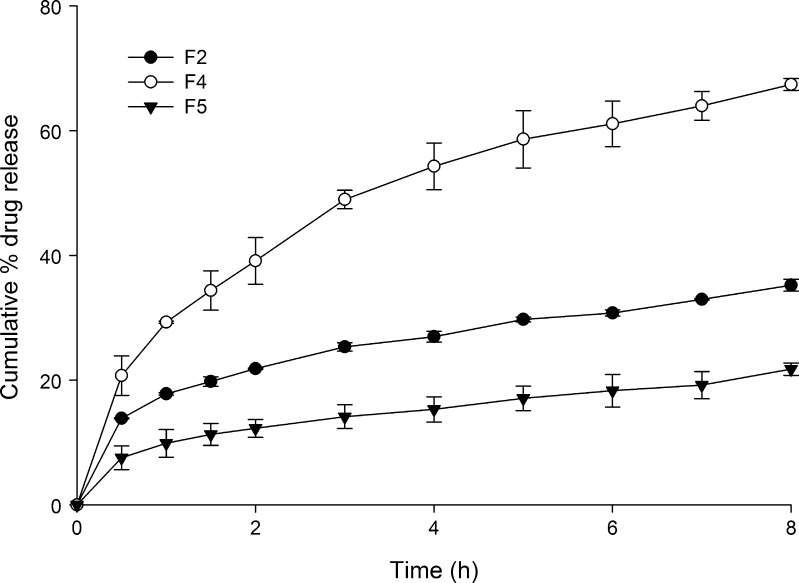
Keeping the amount of tramadol constant at 100 mg in the formulation, amount of wax in each formulation was varied to study its influence on the drug release. The tramadol release from HCSO matrix was retarded significantly (p < 0.05) as the wax concentration was increased (Fig. 2). At the end of 8 h of dissolution study, 67.38 ± 0.95%, 35.22 ± 0.96% and 21.76 ± 0.54% of drug was depleted from tablets prepared by hot fusion, respectively, containing 138, 188 and 238 mg of wax.
Fig. 2.
Effect of HCSO concentration on tramadol release from matrix tablet
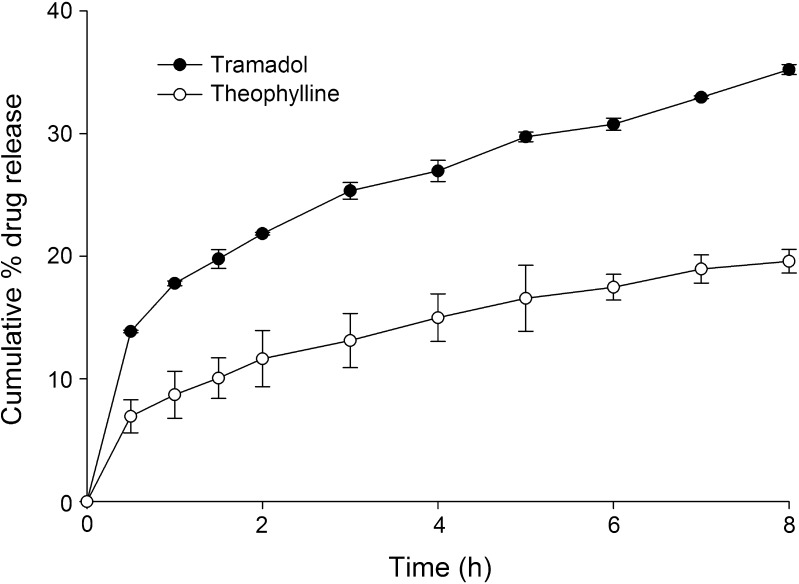
Effect of Drug Solubility on Release
The drug release profiles of tablets containing theophylline or tramadol prepared by hot fusion is shown in Fig. 3. Anhydrous theophylline, a sparingly soluble drug was incorporated in the matrix to evaluate the influence of drug water solubility on release. The release of tramadol was faster than that of theophylline from the formulations indicating that release is directly dependent on the solubility of the drug. At the end of 8 h of dissolution study, 19.08 ± 0.67% and 35.22 ± 0.96% of theophylline and tramadol was released, respectively, from the tablets
Fig. 3.
Influence of solubility of drug on drug release from wax matrix tablet
Effect of Swellable and Water-soluble Excipients on Release
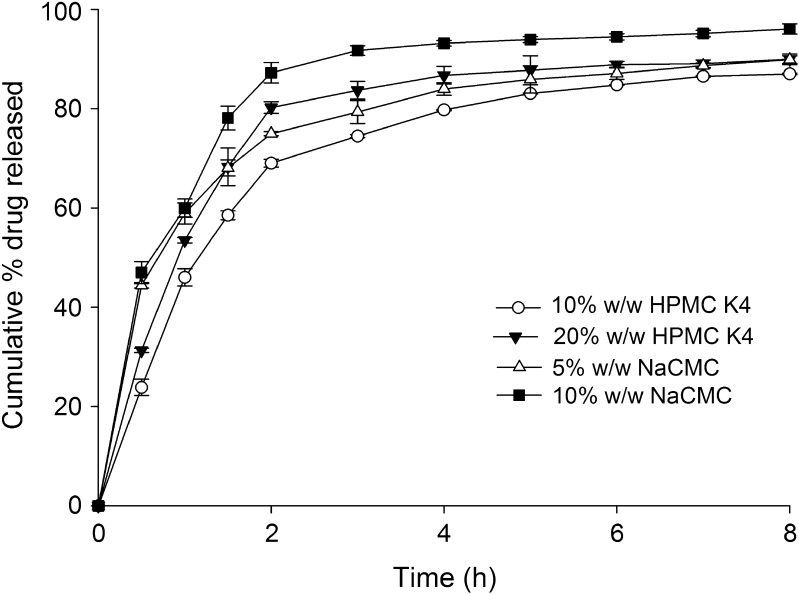
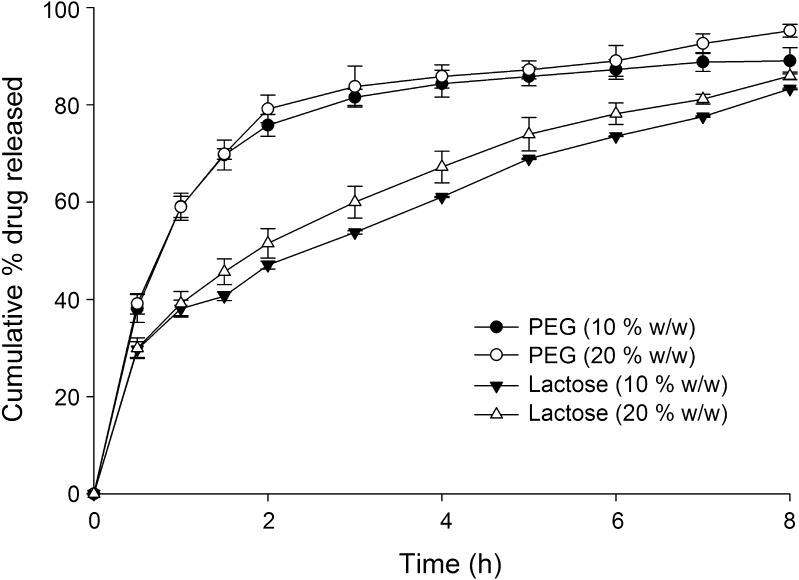
Influence of various excipients on release of tramadol from the HCSO matrix is shown in Figs. 4 and 5. To evaluate the effect of hydrophilic excipients on drug release, tablets were prepared with different ratios of excipients keeping amount of drug and wax constant (Table II). Incorporation of hydrophilic excipients proportionally increased the tramadol release. At the end of 8 h of dissolution study, drug release increased to 96.06 ± 0.30% and 87.01 ± 0.17% on addition of NaCMC (5% w/w) and HPMC K4 (10% w/w), respectively. PEG 4000 and lactose (10% w/w), when added to tablets, increased tramadol release to 89.02 ± 0.69% and 83.26 ± 0.16%, respectively. Though PEG and lactose caused an increase in drug release, no significant (p > 0.05) linear increase in tramadol release was observed with increasing concentrations of PEG or lactose. However, HPMC K4 caused a significant increase in drug release on increasing its concentration in the matrix from 10% to 20% w/w (Fig. 4). Addition of NaCMC at a concentration above 10% w/w had caused tablet to disintegrate within 2 h and hence its concentration in the matrix was restricted to 5% and 10% (w/w).
Fig. 4.
Effect of co-excipients (HPMC K4 and NaCMC) on tramadol release from wax matrix
Fig. 5.
Effect of Lactose and PEG 4000 on tramadol release from matrix tablet
Effect of Surface Active Agent and Their Concentration on Release
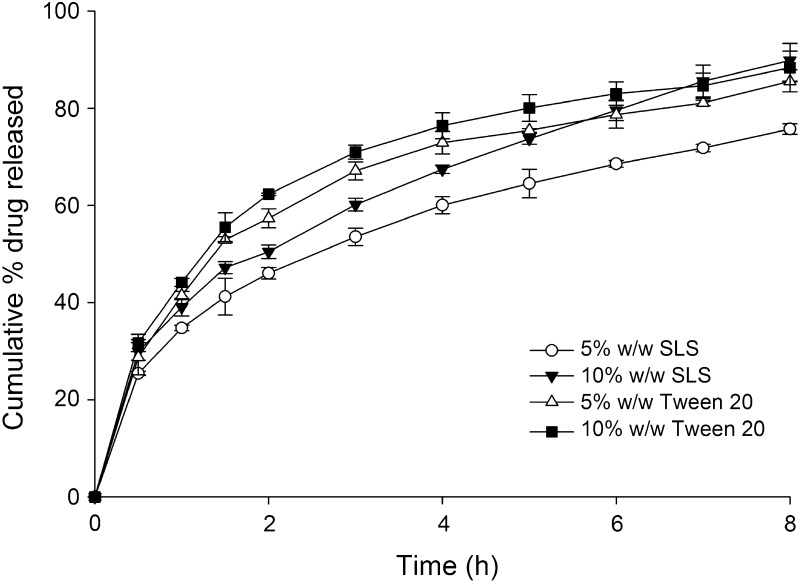
The drug release profiles of HCSO tablets containing either SLS or Tween 20 at different ratios is shown in Fig. 6. Addition of surfactants to HCSO matrix caused marked increase in tramadol release. Tramadol release at the end of 8 h of the dissolution study was increased to 85.54 ± 0.48% and 88.34 ± 0.34% with an addition of 5% and 10% w/w of Tween 20 to the matrix. The other surfactant, SLS caused an increase in release of drug to 75.72 ± 0.85% and 89.84 ± 0.78%, when added at 5% and 10% w/w, respectively.
Fig. 6.
Influence of surfactants on the release of tramadol from HCSO matrix tablets
Effect of Thermal Treatment of Matrix on Release
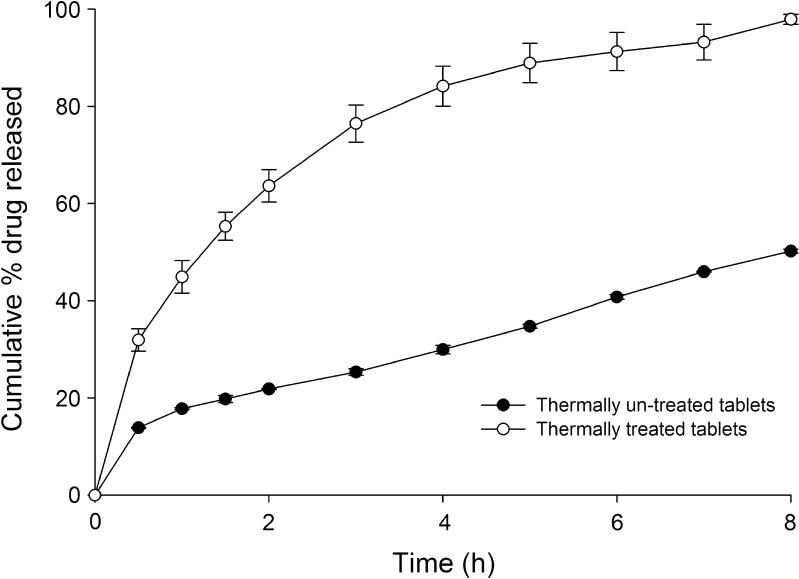
Matrix tablets containing tramadol HCl were thermally treated at 57°C for 1 h. In comparison to non-treated tablets, drug release was faster after thermal treatment (Fig. 7).
Fig. 7.
Drug release profile of thermally treated and untreated formulations
Mechanism of Drug Release
The kinetics and mechanism of drug release was determined using zero-order, first-order, Higuchi’s square root equation (17) and further analysis was performed using Korsemeyer-Peppas equation (18) and Weibull distribution equation (19). Except formulations containing NaCMC (F9 and F10), all others showed best fit to Higuchi’s square root equation (Table III). Formulations F9 and F10 deviated from linearity and exhibited best fit to first-order equation.
Table III.
Drug Release Kinetics and Mechanism from Matrix Tablets
| Formulation | R 2 | n | Weibull equation (b) | ||
|---|---|---|---|---|---|
| Zero order | First order | Higuchi model | |||
| F1 | 0.8396 | 0.8812 | 0.9742 | 0.37 | 0.39 |
| F2 | 0.8053 | 0.8583 | 0.9633 | 0.33 | 0.36 |
| F3 | 0.8662 | 0.9634 | 0.9896 | 0.41 | 0.55 |
| F4 | 0.8481 | 0.9426 | 0.9839 | 0.43 | 0.57 |
| F5 | 0.8517 | 0.8774 | 0.9787 | 0.36 | 0.37 |
| F6 | 0.8543 | 0.8771 | 0.9780 | 0.36 | 0.40 |
| F7 | 0.7186 | 0.9130 | 0.9371 | 0.45 | 0.70 |
| F8 | 0.6102 | 0.7911 | 0.8453 | 0.35 | 0.69 |
| F9 | 0.5900 | 0.8755 | 0.8411 | 0.24 | 0.60 |
| F10 | 0.5371 | 0.8305 | 0.7906 | 0.24 | 0.68 |
| F11 | 0.8254 | 0.9669 | 0.9755 | 0.37 | 0.53 |
| F12 | 0.7864 | 0.9582 | 0.9691 | 0.35 | 0.58 |
| F13 | 0.8420 | 0.9682 | 0.9774 | 0.32 | 0.56 |
| F14 | 0.8440 | 0.9810 | 0.9830 | 0.38 | 0.56 |
| F15 | 0.8361 | 0.9577 | 0.9806 | 0.39 | 0.55 |
| F16 | 0.8680 | 0.9842 | 0.9896 | 0.40 | 0.59 |
| F17 | 0.8353 | 0.9701 | 0.9782 | 0.38 | 0.61 |
| F18 | 0.7859 | 0.9569 | 0.9598 | 0.40 | 0.62 |
All formulations were prepared by hot-fusion method
DISCUSSION
Preparation and Characterization of Tablets
The preparation of the tablets was simple and reproducible. No difficulty was observed during the processing. The prepared tablets were round in shape with smooth surface (visual observation). The results of the physical characterization of tablets indicate that the tablets drug content and weight variation are within the acceptable official limits specified in USP. Further, prepared tablets exhibited an excellent property of withstanding the mechanical stress as indicated by the results of friability and hardness tests.
In Vitro Drug Release Studies
Tramadol release from tablets prepared by hot fusion was significantly (p < 0.05) different from the observed release from tablets prepared by direct compression and wet granulation method. The different release pattern was due to changing matrix internal structure with the process. Results of measure of matrix porosity and tortuosity by some investigators for different matrix give evidence for this (6). Also, their experimental determination reveals that a melt granulation matrix will be less porous and highly tortuous than direct compression matrix. Moreover, during the process of hot fusion, individual drug particle is coated by molten wax which resists faster entry of dissolution fluid through such a hydrophobic matrix. Since the other two techniques involve no heating steps, the only heat that might have generated during compression of physical mixture or granules from wet granulation may not be sufficient enough to melt the wax. Thus, each of the processing method imparts different character to the matrix (20).
The increased drug release with increasing concentration of tramadol is attributed to the matrix porosity in which void space due to air and space occupied by the soluble materials of the formulation create the matrix porosity (1,6,8). Tramadol being freely soluble imparts higher matrix porosity with increasing concentration. The dissolved drug creates pores in the matrix leading to the formation of channels for diffusion of drug across the matrix. According to Higuchi, faster release with increasing tramadol concentration could also be related to corresponding increase in total amount of drug per unit volume of the matrix (17). HCSO imparts hydrophobic character to matrix. At higher wax level, matrix hydrophobicity will increase which resists the entry of water dissolution fluid easily through the matrix resulting reduced drug release. Hence, formulation with highest proportion of HCSO (F5, 238 mg) showed slower drug release than formulations with lower proportion of wax. The tablets were intact with no change in its shape at the end of dissolution study indicating inertness of HCSO matrix and diffusion across the wax matrix could be the associated drug release mechanism (1).
The solubility of theophylline is about 8.3 mg/ml in water, where as tramadol is a freely soluble drug (solubility 30-100 mg/ml). According to Higuchi equation, the drug release from inert matrix system is proportional to square root of solubility, i.e. the higher the water drug solubility, the faster the release of drug from the matrix (17). Further, the observed faster release of tramadol is related to its dissolution and diffusion rate. Drugs with high water solubility have faster dissolution and diffusion than for sparingly soluble drugs (21,22). The difference in the solubility is attributed for the observed faster release of tramadol from the matrix than of theophylline. However, it may not be the only factor which influences drug release.
Solubilisation of hydrophilic excipients in the matrix impart channelling effect in matrix providing extended pathways for the diffusion of dissolution fluid as well as for dissolved drug (10,13). During dissolution process, HPMC builds up an excessively viscous gel around the tablet. The swollen polymer blocks existing pores and prevents further entry of dissolution fluid into the matrix. Moreover, erosion rate of the swollen gel is slow compared with the rate of advancement of swelling front into the glassy tablet core. Thus, the diffusional path length for the drug increases with time, causing the release rate to decrease. However, NaCMC network structure is looser in distilled water causes ionisation of carboxylic group of NaCMC with subsequent repulsion and relaxation of the polymer chains that result in increase in swelling of matrix with time, resulting faster tramadol release (23).
Surfactants when used in the formulation enhance the release of drug by enhancing the hydration of matrix by dissolution medium and increase diffusion of dissolved drug through the system (8). Further, reduced hydrophobic interaction between carrier wax and hydrophilic drug and between the hydrophobic tablet and the water fluid at their interface by surfactant adds for faster release of drug (1).
Thermal treatment of polymeric or wax matrix can retard drug release. Therefore, a formulation with tramadol was subjected to thermal treatment to investigate its effect on drug-retarding property of HCSO. Though hot fusion and thermal treatment involves single-heating procedure the drug release profiles were not same. But both the methods share an increased matrix tortuosity as a common reason for the retarded drug release (6). The reason for the retarded drug release is being related to either formation of a finer but stronger and compact wax network, redistribution of wax through the matrix system or coating on heat treatment (20). The drug release from thermally treated matrix tablet containing tramadol exhibited a faster release than untreated matrix tablets. The result was in contrast with the earlier report where in the release of phenylpropanolamine HCl was decreased from heat treated Compritol® matrix tablet (6). HCSO probably forms an ineffective matrix on heat treatment for controlling highly water-soluble drug. During sintering, water-soluble drug tramadol might have migrated on the surface of the tablet, which on dissolution caused channelling effect rendering the matrix ineffective and facilitating faster release of drug.
Drug Release Mechanism
Release data obtained from the dissolution showed linearity towards square root model described by Higuchi (17) indicating a time-dependent drug release from HCSO tablets. However incorporation of NaCMC caused a deviation from time-dependent to concentration-dependent release kinetics (Table III), probably due to higher solubility of the NaCMC in dissolution fluid. The drug release from the polymeric matrices is substantially influenced by their physico-chemical characters. NaCMC has been shown as a more hydrophilic polymer than other cellulose derivatives (24). This has improved the swelling characters of the NaCMC which caused the observed shift in release kinetics.
The release exponent (n) values determined from the dissolution data was less than 0.5 suggesting the drug release was purely by diffusion through the matrix. This was further substantiated by shape parameter (b) as the determined values was in a range of 0.36-0.70 clearly indicating diffusion as controlling mechanism for drug release from the prepared formulations. Drug release from formulations with uniformly distributed theophylline also followed Higuchi’s square root model and diffusion mechanism. This was rather surprising as theophylline is sparingly soluble and when embedded in a hydrophobic matrix was expected to release following dissolution-controlled mechanism. In previous studies, Cheboyina et al., (7,8) have demonstrated that theophylline existed as homogenously dispersed in glyceryl monostearate and precirol pellets. Moreover, at higher loading (>10% w/w), theophylline existed in crystalline state in the pellets. Further, the release of theophylline from the hydrophilic polyoxyethylene oxide matrices followed dissolution-controlled mechanism (25). Therefore, logically it is expected that drug release follow dissolution-controlled mechanism. Although a definite reason could not be assigned for the observed deviation from the dissolution-controlled mechanism, it might be that a significant proportion of theophylline existed in dissolved state within the lipid matrix. The partitioning of dissolved theophylline to water dissolution fluid might have resulted in diffusion-controlled release mechanism.
CONCLUSION
HCSO can be used as a matrix-forming agent for controlling the release of freely water soluble drugs. The drug release from these matrices can be easily modulated to achieve desired release profile by changing drug or wax concentration. Matrix preparation by hot-fusion method is more effective than other techniques in retarding the release of drug. The results reveal that drug release can be significantly changed simply by the incorporation of suitable release enhancers like surfactants and hydrophilic (PEG 4000, lactose, HPMC or NaCMC) co-excipients.
References
- 1.Li FQ, Hu JH, Deng JX. In vitro controlled release of sodium ferulate from Compritol 888 ATO-based matrix tablets. Int J Pharm. 2006;324:152–157. doi: 10.1016/j.ijpharm.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Tiwari SB, Murthy TK, Pai MR, Mehta PR. Controlled release formulation of tramadol hydrochloride using hydrophilic and hydrophobic matrix system. AAPS PharmSciTech. 2003;4(3):Article 31. [DOI] [PMC free article] [PubMed]
- 3.Martini LG, Coles M, Gravell K, Stephenson S. The use of hydrophobic matrix for the sustained release of a highly water soluble drug. Drug Dev Ind Pharm. 2000;26:79–83. doi: 10.1081/DDC-100100330. [DOI] [PubMed] [Google Scholar]
- 4.Jannin V, Pochard E, Chambin O. Influence of poloxamers on the dissolution performance and stability of controlled-release formulations containing Precirol ATO 5. Int J Pharm. 2006;309:6–15. doi: 10.1016/j.ijpharm.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YE, Schwartz JB. Effect of diluents on tablet integrity and controlled drug release. Drug Dev Ind Pharm. 2000;26:761–765. doi: 10.1081/DDC-100101295. [DOI] [PubMed] [Google Scholar]
- 6.Zhang YE, Schwartz JB. Melt granulation and heat treatment for wax matrix-controlled drug release. Drug Dev Ind Pharm. 2003;29:131–138. doi: 10.1081/DDC-120016720. [DOI] [PubMed] [Google Scholar]
- 7.Cheboyina S, Wyandt CM. Wax- based sustained release matrix pellets prepared by a novel freeze pelletization technique I. Formulation and process variables affecting pellet characteristics. Int J Pharm. 2008;359:158–166. doi: 10.1016/j.ijpharm.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Cheboyina S, Wyandt CM. Wax-based sustained release matrix pellets prepared by a novel freeze pelletization technique II. In vitro drug release studies and release mechanisms. Int J Pharm. 2008;359:167–173. doi: 10.1016/j.ijpharm.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Hamdani J, Moes AJ, Amighi K. Physical and thermal characterisation of Precirol® and Compritol® as lipophilic glycerides used for the preparation of controlled release matrix pellets. Int J Pharm. 2003;260:47–57. doi: 10.1016/S0378-5173(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 10.Parab PV, Oh CK, Ritschel WA. Sustained release from Precirol® matrix. Effect of mannitol and hydroxypropyl methyl cellulose on the release of theophylline. Drug Dev Ind Pharm. 1986;12:1309–1327. doi: 10.3109/03639048609065861. [DOI] [Google Scholar]
- 11.Ghali ES, Klinger GH, Schwartz JB. Thermal treatment of beads with wax for controlled release. Drug Dev Ind Pharm. 1989;15:1311–1328. doi: 10.3109/03639048909062747. [DOI] [Google Scholar]
- 12.Rowe CR, Sheskey PJ, Owen SC. Handbook of pharmaceutical excipient. London: Pharmaceutical press; 2006. [Google Scholar]
- 13.Amaral MH, Lobo JMS, Ferreira DC. Effect of hydroxypropyl methyl cellulose and hydrogenated castor oil on naproxen release from sustained release tablets. AAPS PharmSciTech. 2001;2(2):Article 6. [DOI] [PMC free article] [PubMed]
- 14.O’Neil MJ, Smith A, Heckelman PE. The Merck index. New Jersey: Merck research laboratories; 2001. [Google Scholar]
- 15.Sweetman SC. Martindale—the complete drug reference. London: Pharmaceutical press; 2002. [Google Scholar]
- 16.Saiya D, Bolton D. The use of Precirol® to prepare sustained release tablets of theophylline and quinidine gluconate. Drug Dev Ind Pharm. 1990;16:1963–1969. doi: 10.3109/03639049009023634. [DOI] [Google Scholar]
- 17.Higuchi T. Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 18.Korsmeyer RW, Gurny R, Doelker EM, Buri P, Peppas NA. Mechanism of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]
- 19.Langenbucher F. Linearization of dissolution rate curves by Weibull distribution. J Pharm Pharmcol. 1972;24:979–981. doi: 10.1111/j.2042-7158.1972.tb08930.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang YE, Tchao R, Schwartz JB. Effect of processing methods and heat treatment on the formation of wax matrix tablets for sustained drug release. Pharm Dev Tech. 2001;6:131–144. doi: 10.1081/PDT-100000736. [DOI] [PubMed] [Google Scholar]
- 21.Hamdani J, Moes AJ, Amighi K. Development and evaluation of prolonged release pellets obtained by melt pelletization process. Int J Pharm. 2002;245:67–177. doi: 10.1016/S0378-5173(02)00348-4. [DOI] [PubMed] [Google Scholar]
- 22.Bossard C, Ratismbazafy V, Yiouses DL. Modeling of theophylline compounds release from hard gelatin capsules containing gelucire matrix granules. Drug Dev Ind Pharm. 1991;17:1267–1277. doi: 10.3109/03639049109057296. [DOI] [Google Scholar]
- 23.Kamel AH, Sokar MS, Naggar VF, Gamal SS. Bioadhesive controlled release metronidazole vaginal tablets. Acta Pharm. 2002;52:171–179. [Google Scholar]
- 24.Doelker E. Swelling behavior of water-soluble cellulose derivatives. In: Brannon PL, Harland RS, editors. Absorbent polymer technology. Amsterdam: Elsevier; 1990. pp. 125–145. [Google Scholar]
- 25.Kim C. Drug release from compressed hydrophilic POLYOX-WSR tablets. J Pharmacol Sci. 1995;84:303–306. doi: 10.1002/jps.2600840308. [DOI] [PubMed] [Google Scholar]