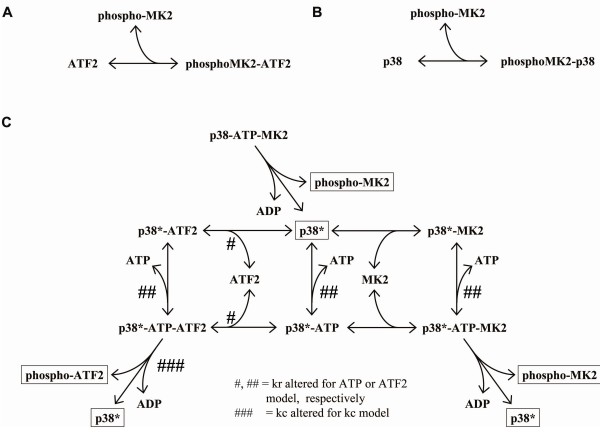
Figure 5.
Alternate kinetic mechanisms. A, substrate inhibition (mechanism #1 in main text): MK2 and ATF2 are allowed to rebind p38 after phosphorylation. B, phospho-MK2 binds ATF2 (mechanism #2 in main text): phosphorylated MK2 is allowed to bind unphosphorylated ATF2 and prevent its interaction with p38. C, altered p38 (mechanisms #3-5 in main text): after phosphorylating MK2, p38 is left in an altered state such that it has either an altered affinity for ATP (mechanism #3 in main text; indicated by #), an altered affinity for ATF2 (mechanism #4 in main text; indicated by ##) or an altered catalytic rate for ATF2 (mechanism #5 in main text; indicated by ###).