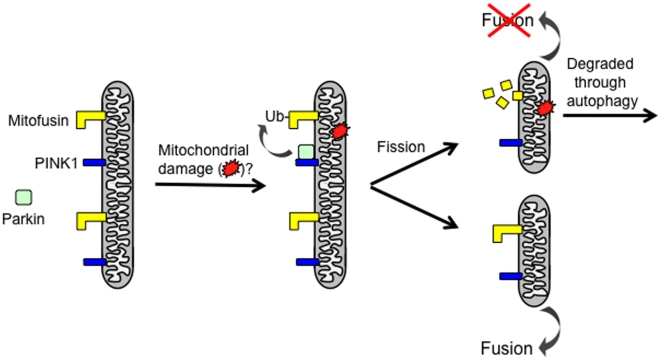
Figure 5. A potential model by which the PINK1/Parkin pathway promotes mitochondrial fragmentation and turnover.
Previous work has shown that PINK1 localizes to the outer mitochondrial membrane with its kinase domain facing the cytoplasm [28], [39], where it is required to recruit Parkin selectively to damaged mitochondria to promote the autophagic clearance of these mitochondria [27], [28], [29], [30], [31]. Our model postulates that upon Parkin recruitment to damaged portions of the mitochondrial reticulum, it ubiquitinates (Ub) the mitochondrial fusion-promoting factor Mitofusin, thus tagging it for degradation, or otherwise inactivating its fusion-promoting activity. Subsequent Drp1-dependent mitochondrial fission would yield a damaged mitochondrial product that is deficient in Mitofusin, and thus unable to re-enter the mitochondrial network. These small damaged mitochondrial fission products would instead be targeted for autophagic degradation. Although our figure depicts a model in which Parkin binds prior to mitochondrial fission, Parkin could also be recruited to damaged mitochondria following fission. An alternative model is that the ubiquitination of Mitofusin serves as a signal for the turnover of damaged mitochondria. These possible models are not mutually exclusive.