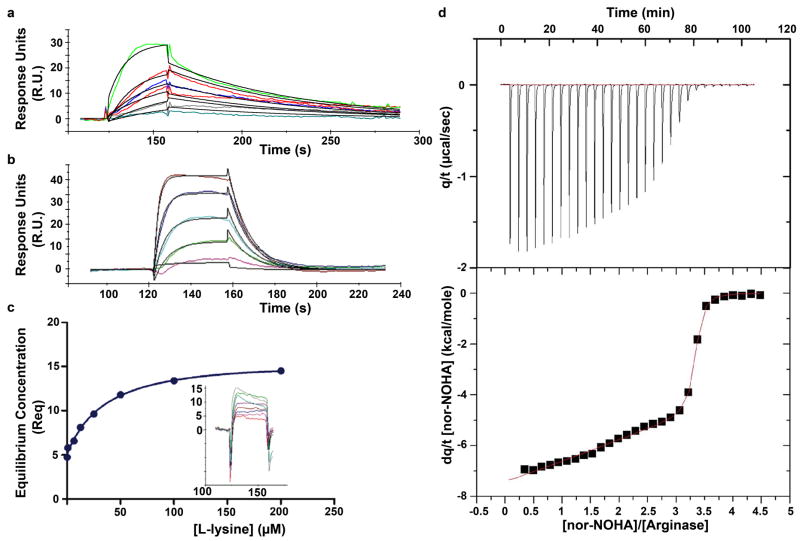
Figure 1.
(a) Sensorgram showing the interaction of nor-NOHA with human arginase I, yielding Kd = 517 nM. (b) Sensorgram showing the interaction of NOHA with human arginase I, yielding Kd = 3.6 μM. (c) Plot of equilibrium concentrations (Req) determined by surface plasmon resonance for the complexation of human arginase I with L-lysine yields Kd = 13.1 μM; binding kinetics are too rapid to facilitate direct determination of association and dissociation rate constants (inset). (d) Isothermal titration calorimetry of nor-NOHA binding to human arginase I. Shown are the raw data obtained by titration of 0.036 mM arginase with 30 × 5 μL injections of 1.5 mM nor-NOHA (top). The area of each peak is integrated and plotted against [nor-NOHA]/[arginase] (bottom); nonlinear least-squares fitting of the data to a two-site model (solid line) yields Kd values of 51 nM and 47 nM.