Abstract
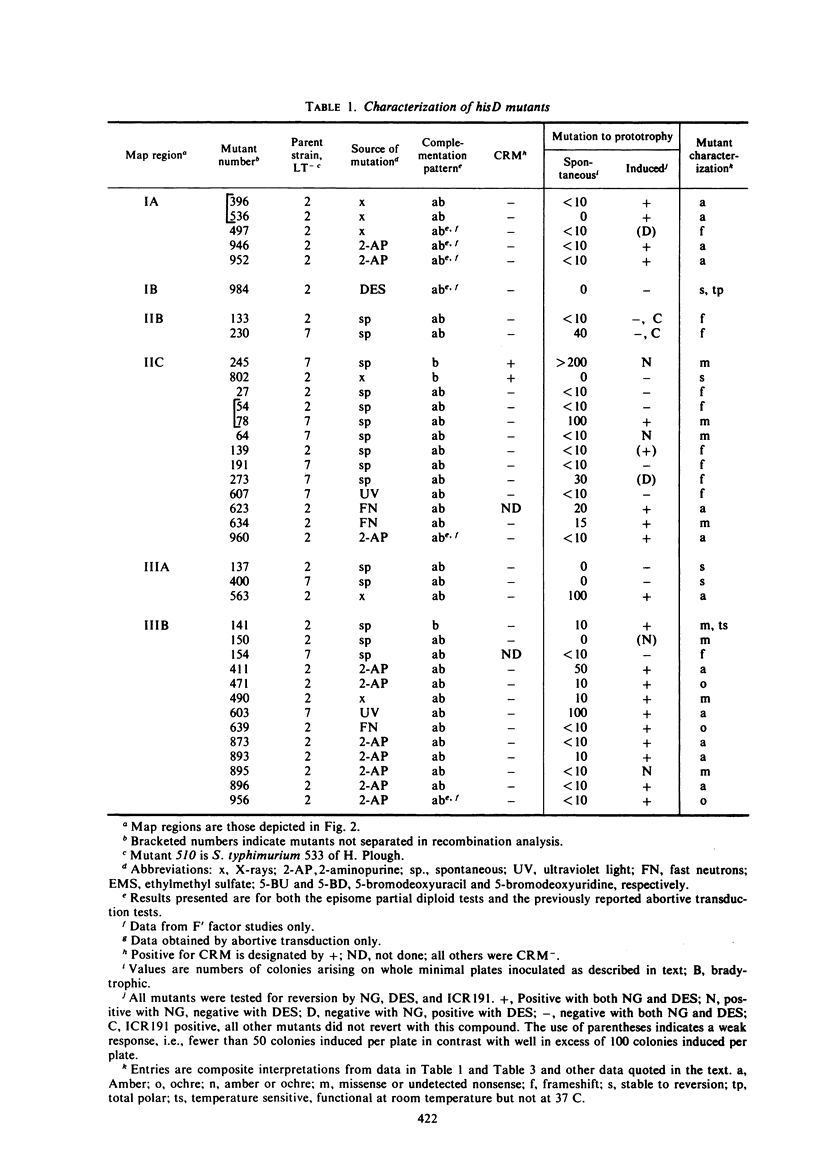
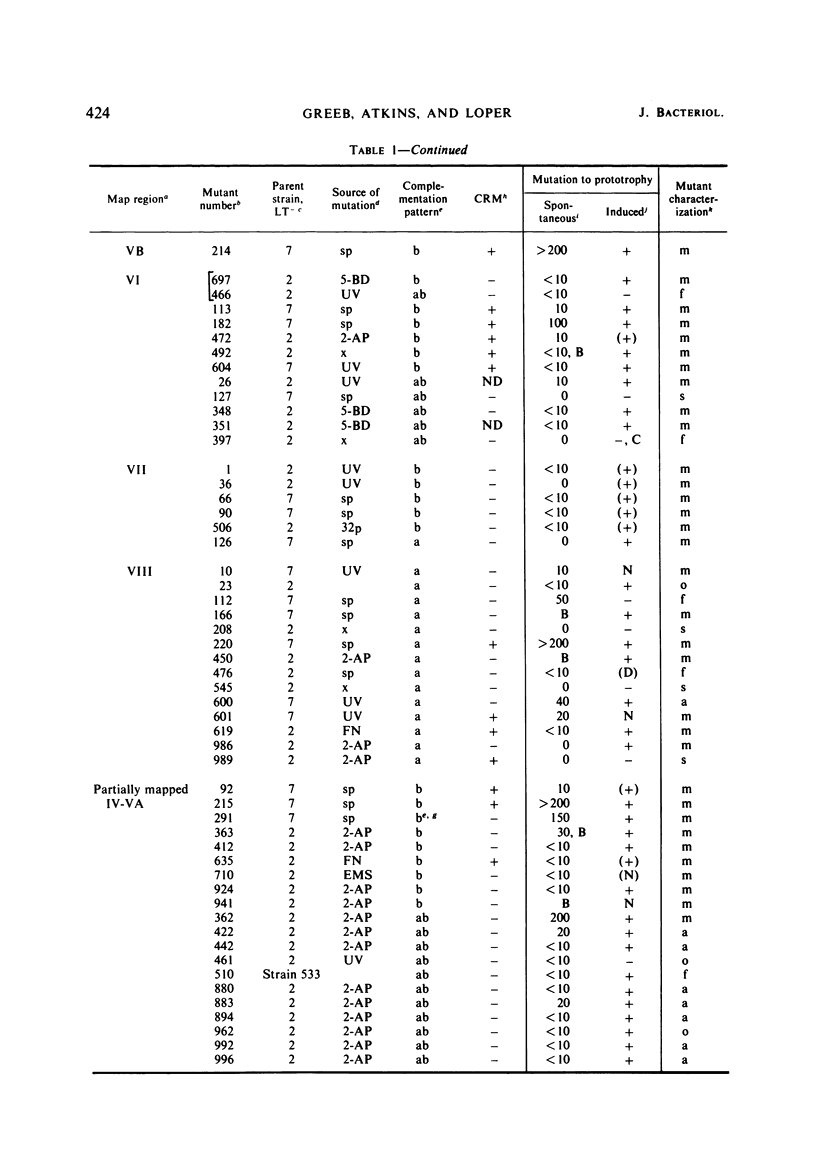
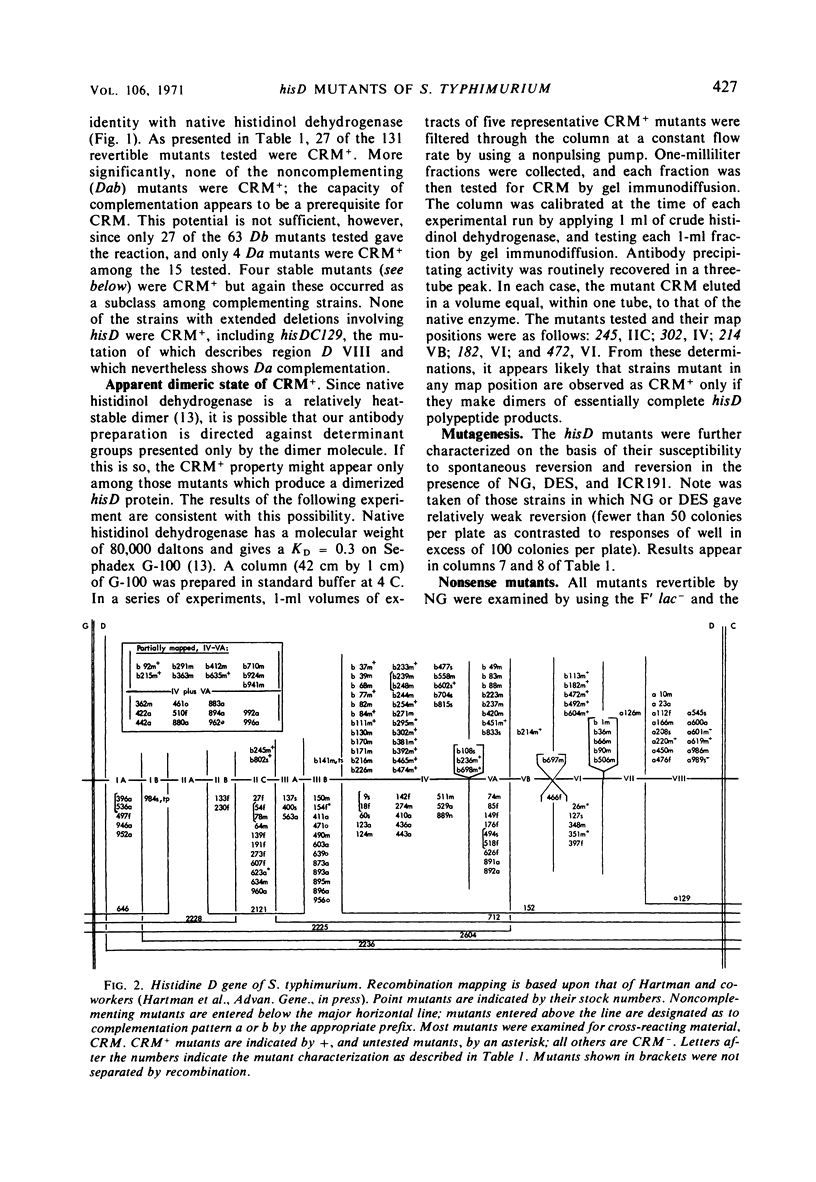
A multidisciplinary analysis has been applied to over 150 hisD mutants of Salmonella typhimurium in a study of gene-enzyme relationship. The mutants were examined for production of immunologically cross-reacting material by using antibody to purified histidinol dehydrogenase, and for genetic complementation by using a set of F′ factors bearing Escherichia coli hisD complementing mutants. Classifications as to missense, nonsense, frameshift, or deletion mutant are proposed on the basis of mutagenesis and suppression tests. For the suppression tests the mutants were examined both by a simultaneous suppression technique and by testing for response to E. coli F′ factors bearing a recessive lethal amber and a recessive lethal ochre suppressor. The data are interpreted in relation to the position of the mutations in the recombination and complementation maps and in relation to the known composition of histidinol dehydrogenase. The gene hisD appears to be single cistron for the production of a single biosynthetic polypeptide.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., GARRY B., HERZENBERG L. A. The genetic control of the enzymes of histidine biosynthesis in Salmonella typhimurium. J Gen Microbiol. 1960 Apr;22:369–378. doi: 10.1099/00221287-22-2-369. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Loper J. C. Transcription initiation in the histidine operon of Salmonella typhimurium. Proc Natl Acad Sci U S A. 1970 Apr;65(4):925–932. doi: 10.1073/pnas.65.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H., Brammar W. J., Yanofsky C. Spontaneous and ICR191-A-induced frameshift mutations in the A gene of Escherichia coli tryptophan synthetase. J Bacteriol. 1968 Nov;96(5):1672–1679. doi: 10.1128/jb.96.5.1672-1679.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz D., Hushon J. M., Whitfield H. J., Jr, Roth J., Ames B. N. Procedure for identifying nonsense mutations. J Bacteriol. 1968 Jul;96(1):215–220. doi: 10.1128/jb.96.1.215-220.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzicker T., Zipser D. A mutation which creates a new site for the re-initiation of polypeptide synthesis in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1968 Dec;38(3):305–314. doi: 10.1016/0022-2836(68)90388-4. [DOI] [PubMed] [Google Scholar]
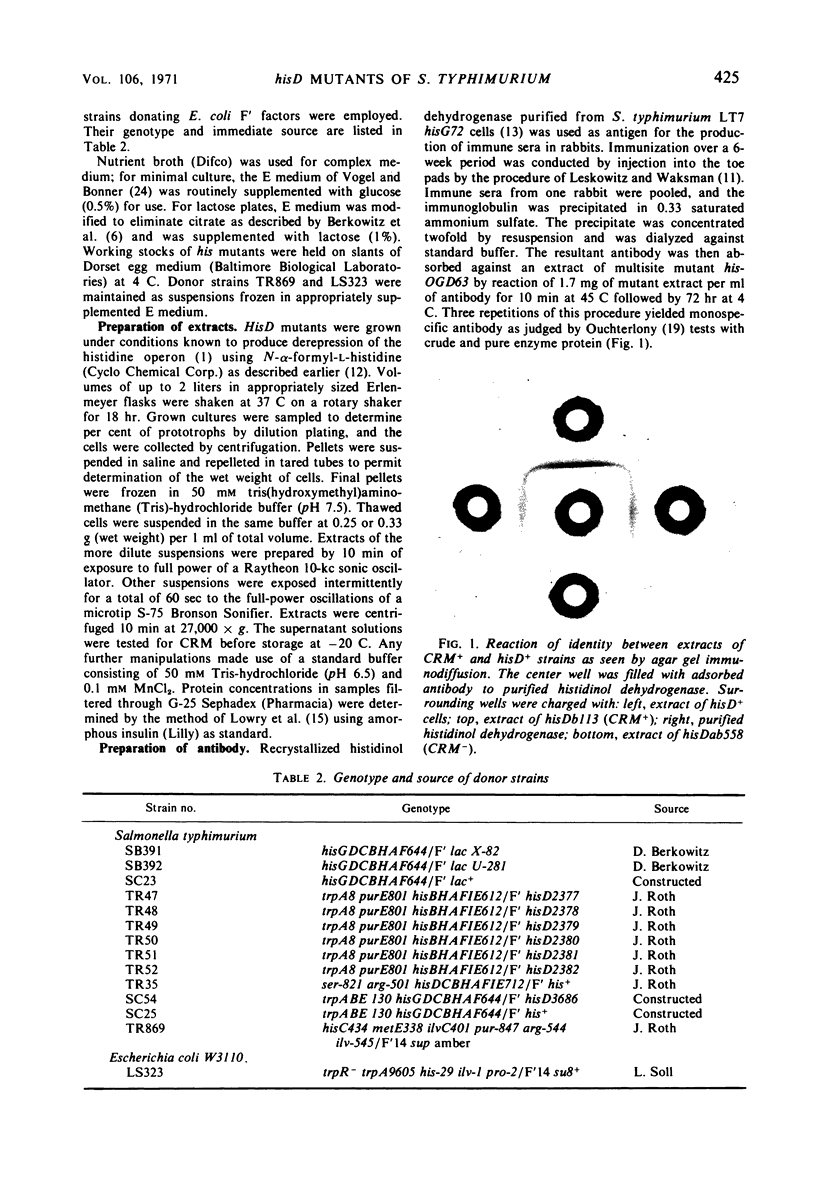
- LESKOWITZ S., WAKSMAN B. H. Studies on immunization. 1. The effect of route of injection of bovine serum albumin in Freund adjuvant on production of circulating antibody and delayed hypersensitivity. J Immunol. 1960 Jan;84:58–72. [PubMed] [Google Scholar]
- LOPER J. C. Enzyme complementation in mixed extracts of mutants from the Salmonella histidine B locus. Proc Natl Acad Sci U S A. 1961 Sep 15;47:1440–1450. doi: 10.1073/pnas.47.9.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPER J. C., GRABNAR M., STAHL R. C., HARTMAN Z., HARTMAN P. E. GENES AND PROTEINS INVOLVED IN HISTIDINE BIOSYNTHESIS IN SALMONELLA. Brookhaven Symp Biol. 1964 Dec;17:15–52. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langridge J., Campbell J. H. Classification and intragenic position of mutations in the beta-galactosidase gene of Escherichia coli. Mol Gen Genet. 1969;103(4):339–347. doi: 10.1007/BF00383484. [DOI] [PubMed] [Google Scholar]
- Loper J. C. Histidinol dehydrogenase from Salmonella typhimurium. Crystallization and composition studies. J Biol Chem. 1968 Jun 25;243(12):3264–3272. [PubMed] [Google Scholar]
- Martin R. G., Silbert D. F., Smith W. E., Whitfield H. J., Jr Polarity in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):357–369. doi: 10.1016/0022-2836(66)90104-5. [DOI] [PubMed] [Google Scholar]
- Oeschger N. S., Hartman P. E. ICR-induced frameshift mutations in the histidine operon of Salmonella. J Bacteriol. 1970 Feb;101(2):490–504. doi: 10.1128/jb.101.2.490-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R. UGA nonsense mutations in Salmonella typhimurium. J Bacteriol. 1970 May;102(2):467–475. doi: 10.1128/jb.102.2.467-475.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethal nonsense suppressor in Escherichia coli which inserts glutamine. Nature. 1969 Sep 27;223(5213):1340–1342. doi: 10.1038/2231340a0. [DOI] [PubMed] [Google Scholar]
- Soll L., Berg P. Recessive lethals: a new class of nonsense suppressors in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jun;63(2):392–399. doi: 10.1073/pnas.63.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanemura S., Yourno J. Frameshift revertant of Salmonella typhimurium producing histidinol dehydrogenase with a sequence of four extra amino acid residues. J Mol Biol. 1969 Dec 28;46(3):459–466. doi: 10.1016/0022-2836(69)90189-2. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Whitfield H. J., Jr, Martin R. G., Ames B. N. Classification of aminotransferase (C gene) mutants in the histidine operon. J Mol Biol. 1966 Nov 14;21(2):335–355. doi: 10.1016/0022-2836(66)90103-3. [DOI] [PubMed] [Google Scholar]
- Wolf R. E., Jr, Loper J. C. The differential inactivation of histidinol dehydrogenase from Salmonella typhimurium by sulfhydryl reagents. J Biol Chem. 1969 Nov 25;244(22):6297–6303. [PubMed] [Google Scholar]
- Yourno J. Composition and subunit structure of histidinol dehydrogenase from Salmonella typhimurium. J Biol Chem. 1968 Jun 25;243(12):3277–3288. [PubMed] [Google Scholar]
- Zipser D. Polar mutations and operon function. Nature. 1969 Jan 4;221(5175):21–25. doi: 10.1038/221021a0. [DOI] [PubMed] [Google Scholar]



