FIGURE 1.
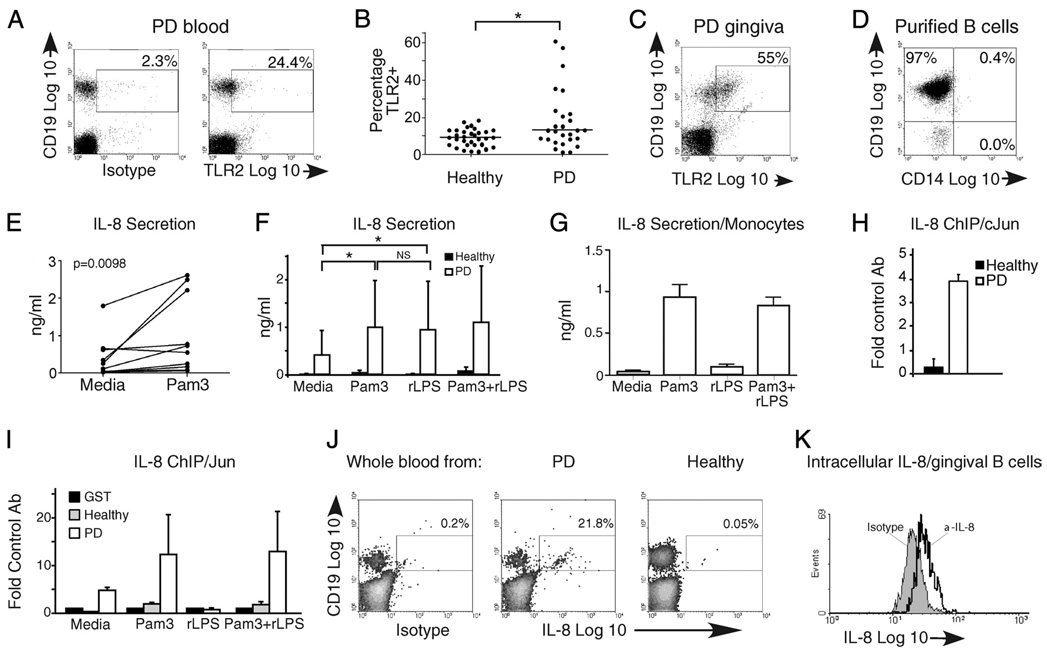
An elevated percentage of CD19+ B cells from PD patients express functional TLR2 and secrete IL-8. A, Representative flow cytometric plot of B cells in peripheral blood from a PD patient stained for surface TLR2 expression relative to isotype control. A low percentage of CD19+TLR2+ B cells in blood of healthy donors has been published (30). B, Analysis of percentage and median percentage (horizontal line) of TLR2+ B cells in 32 healthy and 27 PD donors. Each point represents one donor. The percentages of TLR2+ B cells are significantly different (p= 0.048 by Mann-Whitney U test) between the two cohorts. Medians were: healthy = 8.97%, PD = 12.5% TLR2+ B cells. C, Flow cytometric plot showing percentage of CD19+TLR2+ B cells in surgically excised gingival tissue from PD patients. One of three similar results is shown. D, Representative reanalysis of isolated B cells from peripheral blood to demonstrate purity in samples subjected to biochemical analyses. The CD19−CD14− population (lower left) was predominantly CD3+ T cells (not shown). E, IL-8 secretion by peripheral blood B cells from 10 PD patients incubated in the absence vs presence of the TLR2 ligand Pam3CSK4. A paired t test established significance (p= 0.0098, shown in upper left). Each set of paired points shows results from a single donor under unstimulated (left) or stimulated (right) conditions. F, IL-8 secretion in B cells from PD (open bars) and healthy (filled bars) donors in the absence or presence of TLR2 (Pam3) and/or TLR4 (rLPS) ligand. Shown is average and SEM of 11 PD donors. *p < 0.05; NS, p > 0.1. G, IL-8 secretion by human monocytes stimulated for 24 h as indicated. Shown is average and SD of determinations from three donor samples. H, ChIP measuring constitutive association between the IL-8-activating transcription factor c-Jun and the IL-8 promoter in fresh ex vivo B cells from healthy (filled bar) or PD (open bar) donors. I, ChIP measuring c-Jun association with the IL-8 promoter in B cells from healthy (gray bars) or PD (open bars) donors incubated for 6 h with stimulus indicated on the x-axis. H and I show fold increase in DNA amplification in samples precipitated with the indicated Ab compared with an irrelevant Ab (anti-GST). Error bars show range from two to three donors for each treatment. J, Flow cytometry measuring intracellular IL-8 in B cells from PD or healthy donors as indicated. Isotype control was performed on the PD sample shown in the middle panel. Note that permeabilization decreases the CD19 signal nonspecifically. Percentage of IL-8-positive cells in the CD19+ gate is indicated. K, Flow cytometry measuring intracellular IL-8 in B cells from surgically excised gingiva from PD patients. Cells were gated on CD19. Forty-four percent of the 31,000 cells analyzed were IL-8+ in this sample. J and K represent independent determinations from three donors.