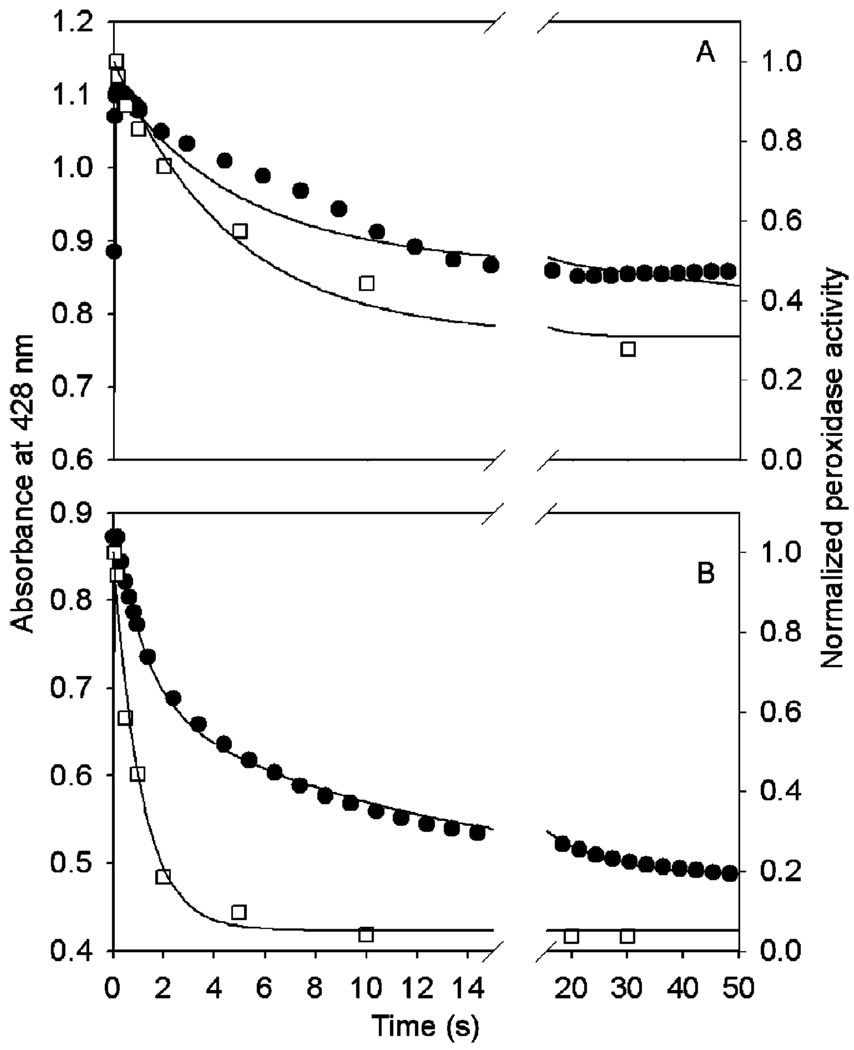
FIGURE 9.
Comparison of observed kinetics of A428 (●) and normalized remaining peroxidase activity (□) during reaction of Fe-PGHS-1 with 20 equiv of EtOOH (A) or 15-HPETE (B) from the experiments in Figure 6 and Figure 7 with computer simulations (─) generated as described in Experimental Procedures. The optimal values of rate constants for reaction with EtOOH are listed in Table 1, and the following parameters were used: ε1 = 0.063 µM−1 cm−1, ε2 = 0.102 µM−1 cm−1, ε3 = 0.080 µM−1 cm−1, ε4 = 0.061 µM−1 cm−1, and Residual = 0.34. The optimal values of rate constants for reaction with 15-HPETE are listed in Table 1, and the following parameters were used: ε1 = 0.060 µM−1 cm−1, ε2 = 0.091 µM−1 cm−1, ε3 = 0.068 µM−1 cm−1, ε4 = 0.049 µM−1 cm−1, and Residual = 0.05.