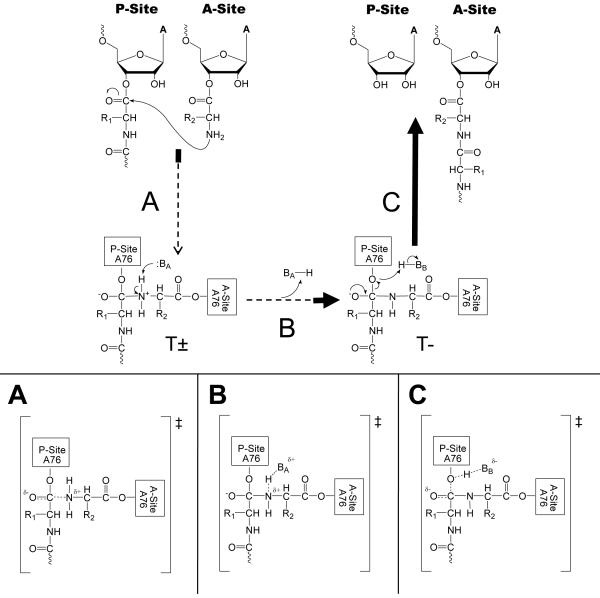
Figure 3.
Stepwise mechanism with two intermediates (T± and T−). The relative energetics of intermediates and TS are arbitrary. The bolded portions of the arrows represent areas where a zero βnuc is consistent for each transition. (A) Possible TS if nucleophilic attack determines the reaction rate. (B) Possible TS if α-amine proton transfer following T± formation determines the reaction rate. (C) Possible TS if T- tetrahedral intermediate breakdown determines the reaction rate.