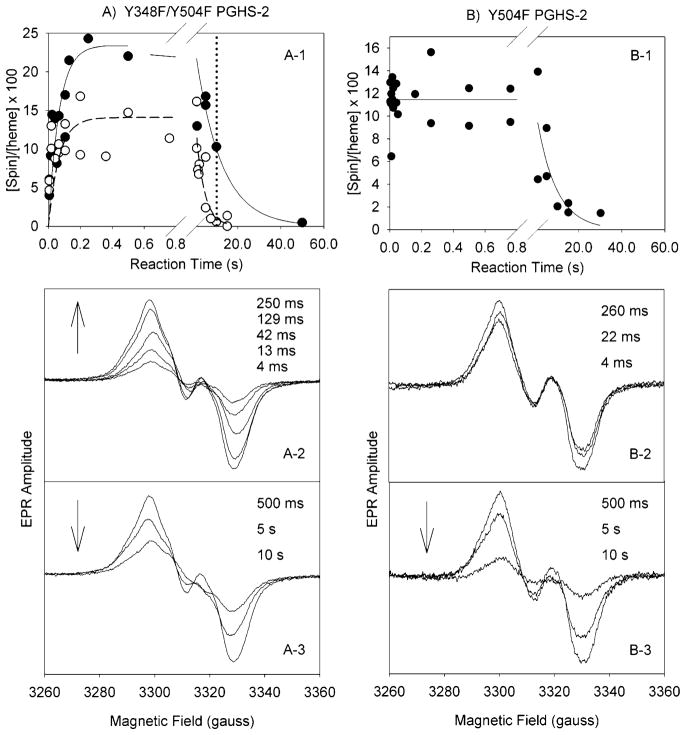
Figure 2.
Tyrosyl radical kinetics during the reaction of Y348F/Y504F PGHS-2 and Y504F PGHS-2 with EtOOH. (A) The double mutant (68 μM heme) in 100 mM KPi (pH 7.2), 50 μM phenol, 0.04% octyl glucoside, and 10% glycerol was reacted at room temperature with 15 equiv of EtOOH. (A-1) Time course of Y348F/Y504F tyrosyl radical intensity as determined by double integration of the EPR signals (●) in experiments with two separate enzyme preparations and time course for the PGHS-2 Y148F/Y348F/Y404F/Y504F quadruple mutant (○) (25). (A-2 and A-3) EPR spectra from the double mutant reaction samples freeze trapped at the indicated times. (B) Y504F PGHS-2 (48 μM heme) was reacted with 15 equiv of EtOOH at room temperature (25). (B-1) Time course of radical intensity determined by double integration. (B-2 and B-3) EPR spectra from the Y504F mutant reaction samples freeze trapped at the indicated times.