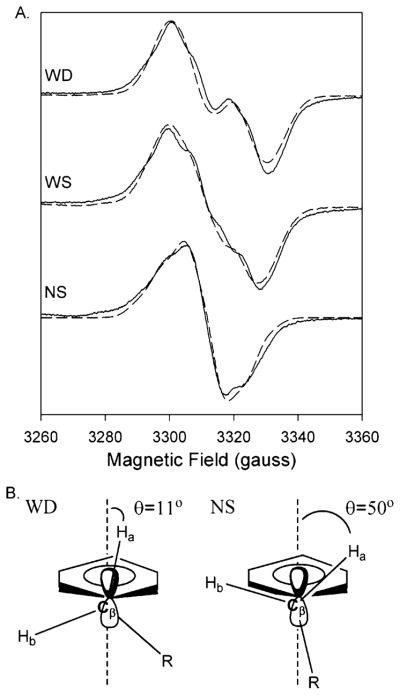
Figure 3.
(A) Experimental (—) and simulated (– – –) EPR spectra of the doublet, wide singlet (WS), and narrow singlet (NS) EPR signals from Y348F/Y504F PGHS-2 reacted with peroxide. The doublet was observed after reaction of the double mutant (38 μM heme) with 15 equiv of EtOOH for 104 ms. The simulated spectrum represents the sum of 80% of the Y504F WD simulated spectrum and 20% of the NS simulated spectrum. The WS was observed after reaction of the double mutant (15 μM heme) with 15 equiv of EtOOH on ice for ~10 s. The wide singlet simulation represents the sum of 48% simulated Y504F WD and 52% simulated NS. The NS spectrum was recorded after preincubation of the double mutant (15 μM heme) with 5 equiv of nimesulide and subsequent reaction with 15 equiv of EtOOH for 10 s on ice. Parameters for simulation of the Y504F WD and NS spectra are given in Table 3. (B) Structural diagrams of the proposed conformations of the β-methylene groups of tyrosyl radicals that give rise to the WD and NS spectra.