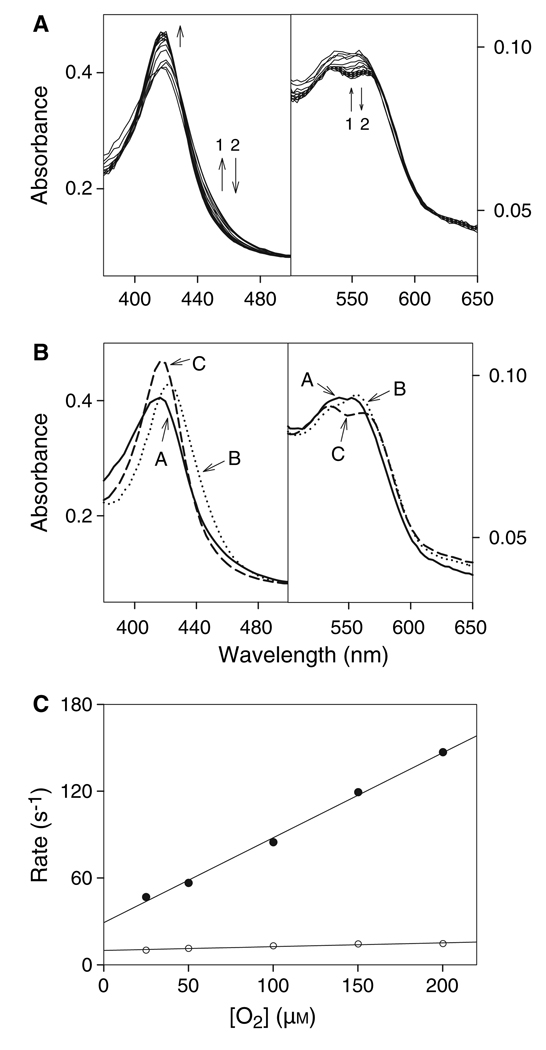
Fig. 1.
Stopped-flow study of ferrous PGIS reaction with oxygen. (A) Rapid scan absorbance spectral changes for the reaction of ferrous PGIS (5 µm) with oxygenated buffer (100 µm) at 4 °C. Spectra were recorded at 0.0013, 0.0064, 0.014 and 0.0127 s, and then at increments of 0.026-s intervals until 1 s of reaction time had been monitored. Arrows show the directions of spectral changes with increasing time, and numbers indicate the orders of the signal change. (B) Spectral intermediates resolved by global analysis using the sequential model of A ↔ B→C (species A, solid line; species B, dotted line; species C, dashed line). (C) Plots of the observed rate constants for the first phase (filled circles) and the second phase (open circles) versus oxygen concentration. Experiments were carried out by mixing ferrous PGIS (5.0 µm) with various concentrations of oxygenated buffer (25–200 µm).