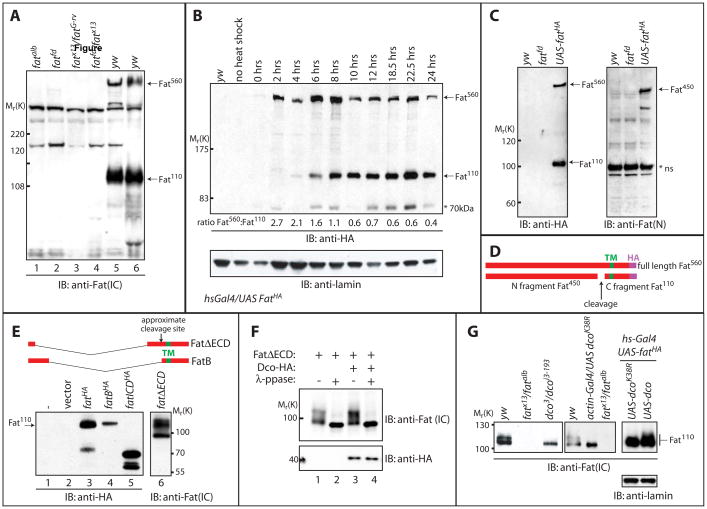
Figure 1. Fat processing generates a 110kDa form, which displays altered electrophoretic mobility in dco mutants.
A) Larval extracts from yw, fatalb, fatfd, fatfd/fatG-rv, fatfd/fatx13 were subjected to SDS-PAGE and analyzed by Western blotting with α-Fat (IC) antibody. 560kDa and 110kDa bands (indicated by arrows) recognized in yw (wildtype) extracts by the α-Fat (IC) antibody are absent from all fat extracts.
B) In vivo pulse chase analysis of FatHA. Larvae bearing a C terminally HA-tagged UAS-Fat transgene and hs-gal4 driver (hs-Gal4; UAS-FatHA) were subjected to a brief heat shock at 37°C and then returned to 18°C. Western blotting with α-HA of larval extracts from these animals at the indicated time points following heat shock revealed that Fat is first produced as a 560kDa protein (Fat560; top arrow). A band migrating at 110kDa (Fat110; lower arrow) however appears 4–6 hours post heat shock, and this band predominates after 10hrs (further emphasized by quantitation of the ratio of Fat560 to Fat110 below blot). Immunoblotting with α-lamin (lower panel) serves as a loading control. A 70kDa band (indicated with an asterisk) is also inconsistently detected with α-Fat (IC) antibody.
C) Larval extracts from yw, fatfd, and hs-Gal4;UAS-fatHA animals were subjected to SDS-PAGE and analyzed by Western blotting with α-Fat (N) antibody. This antibody detected a 450kDa band (Fat450; top arrow, right panel), while α-HA recognized a 560kDa band (Fat560; top arrow, left panel) and a 110kDa band (Fat110; bottom arrow, left panel). A non-specific band in all extracts (*ns) is also detected with α-Fat (N).
D) Schematic of Fat protein generated from expression of the UAS-fatHA transgene. The transmembrane domain (TM), C-terminal HA tag, and fragments generated upon cleavage of full length Fat (Fat560) are indicated.
E) Extracts from Drosophila S2 cells expressing HA-tagged full length Fat (lane 3), the Fat intracellular domain (ICD, lane 5), or versions of Fat encompassing both the transmembrane and intracellular domains (FatB and FatΔECD which vary in their extracellular sequence, lanes 4 and 6 respectively) were subjected to SDS-PAGE and analyzed by Western blotting with α-HA antibody. Expression of full-length Fat-HA generates Fat110, as well as Fat560 (not shown on this portion of the gel) while Fat ICD migrates at ~70kDa and FatB and FatΔECD at ~110kDa, suggesting Fat110 includes the transmembrane domain.
F) Lysates from Drosophila S2 cells expressing FatΔECD with or without HA-tagged Dco were divided. One half was treated with lambda phosphatase and the other mock treated. Samples were subjected to SDS-PAGE and Western blotting using α-HA or α-Fat (IC) antibodies.
G) Larval extracts from yw, dco3/dcoi3-193, fatAlb/fatx13, dcoK38R expressing, dco and fatHA co-expressing or dcoK38R and fatHA co-expressing larvae were analyzed by immunoblotting with α-Fat (IC) antibody. The Fat110 doublet is reduced to a single band in extracts from dco3/dcoi3-193 larvae and larvae expressing dcoK38A while overexpression of dco causes a decrease in the mobility of co-overexpressed Fat, visualized as an increase in the slower migrating form of the doublet. Immunoblotting with a-lamin (lower panel) serves as a loading control.