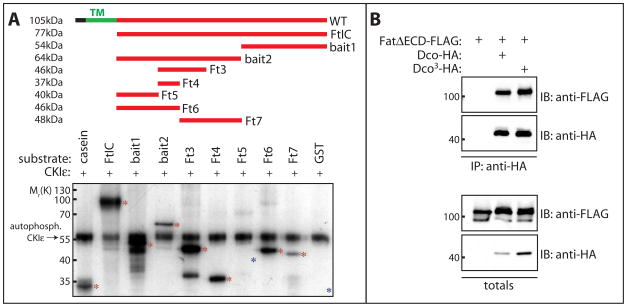
Figure 3. CKIε phosphorylates Fat and binding to Fat is not affected by the Dco3 mutation.
A) Recombinant GST-Fat fusion proteins (100ng) were incubated with human CKIε (50ng) and γ-32P-ATP. Casein was included as a positive control (lane 1). Phosphorylation of Fat fragments was analyzed by SDS-PAGE and autoradiography. The position of migration of input proteins is indicated with asterisks. Auto-phosphorylated CKIε migrates at ~55kDa. All fragments except Fat5 and GST (lanes 7 and 10 respectively, indicated with blue asterisks) showed robust phosphorylation, indicating multiple sites are phosphorylated by CKIε.
B) Both Dco and Dco3 interact with Fat. Lysates from Drosophila S2 cells expressing HA-tagged Dco or Dco3 together with FLAG-tagged FatΔECD were subjected to immunoprecipitation with anti-HA antibody and immunoprecipitates analyzed by immunoblotting with the indicated antibodies.