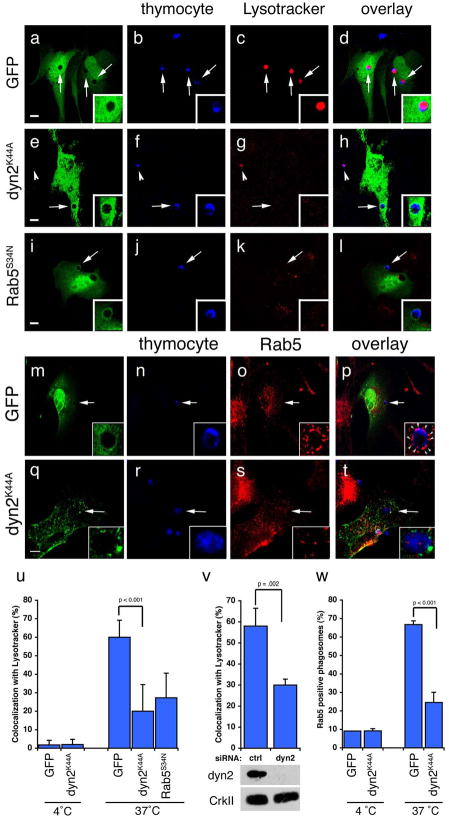
Figure 6. Dynamin is required for maturation of engulfed apoptotic cells into Rab5-coated endosomes.
Arrows and arrowheads indicate apoptotic cells or protein recruited around engulfed apoptotic cells in phagosomes. Neither Dyn2K44A nor Dyn2RNAi had any obvious effect on staining of endogenous endosomal/lysosomal structures with Lysotracker Red or Rab5. Error bars represent s.d. Scale bar, 10 Îm.
(aâl) NIH/3T3 fibroblasts transfected with GFP (aâd), HA-Dyn2K44A (eâh), or GFP-Rab5S34N (iâl) were incubated with apoptotic thymocytes in the presence of Lysotracker Red to determine the efficiency of phagosome maturation. In the majority of GFP-transfected cells, internalized apoptotic thymocytes (arrows) co-stained with Lysotracker red (d, inset); cells transfected with Dyn2K44A (h, inset) or Rab5S34N (l, inset) showed decreased numbers of engulfed thymocytes co-staining with Lysotracker. Apoptotic cells incubated at 4 ÂC with phagocytes did not stain strongly with Lysotracker Red (u, Supplementary Figure S9). HA-Dyn2K44A expressing cells were stained with anti-HA and an Alexa 488-conjugated secondary antibody for visualization of transfected cells.
(mât) Apoptotic cells were incubated with NIH/3T3 fibroblasts, transfected with either GFP alone (as control) or HA-Dyn2K44A and the localization of endogenous Rab5 was monitored. The majority of engulfed apoptotic thymocytes inside GFP-transfected cells were in endosomes coated with Rab5 (o, arrowheads, p, inset). In Dyn2K44A transfected cells, phagosome maturation was disrupted and decreased numbers of engulfed thymocytes were in Rab5 coated endosomes (s, t, arrowhead, inset). Apoptotic cells incubated at 4 ÂC with phagocytes did not stain strongly for Rab5 (w, Supplementary Figure S9)
(u) Quantitation of experiment shown in (aâl). 4 ÂC n=20 (2 experiments), 37 ÂC, n=60 (3 experiments).
(v) NIH/3T3 fibroblasts were electroporated with a control siRNA or dynamin-2 (dyn2) siRNA and assayed as in (aâl). Dyn2siRNA cells showed decreased co-localization with Lysotracker as compared to controlsiRNA cells. n=50 (2 experiments).
(w) Quantitation of experiment shown in (mât). 4 ÂC n=20 (2 experiments), 37 ÂC, n=60 (4 experiments).