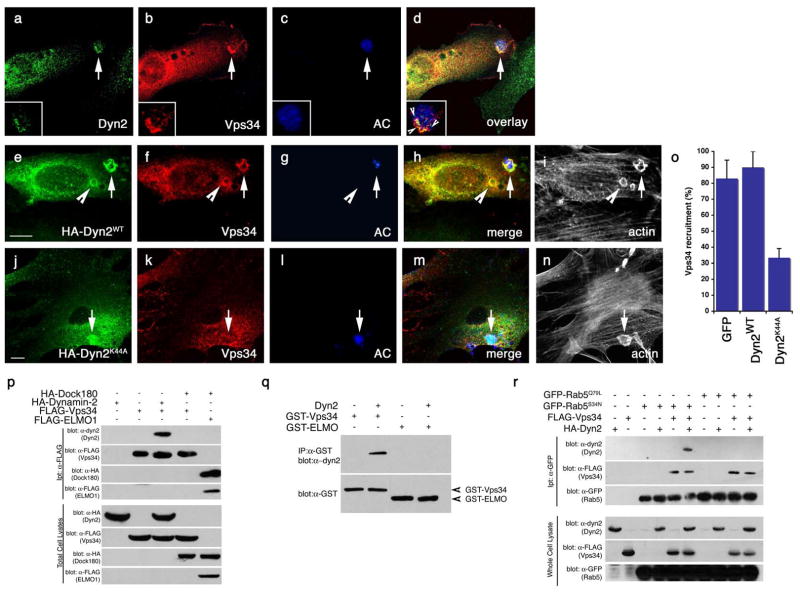
Figure 8. Vps34 functions downstream of Dyn2 and mediates the interaction between Dyn2 and Rab5.
Arrows indicate localization of phagocytic cup or co-localizaiton of indicated proteins. Size bar, 10 Îm. Apoptotic cells were stained with CMHC, whose fluorescence becomes less detectable when the apoptotic cells are engulfed (g, arrowhead).
(aâd) Apoptotic Jurkat cells were incubated with NIH/3T3 fibroblasts transiently transfected with FLAG-Vps34 and the localization of endogenous Dyn2 (a) and FLAG-Vps34 (b) in phagocytic cups containing apoptotic cells (c) were monitored. Both proteins co-localize in the phagocytic cup (d, overlay, arrow)
(eân) Apoptotic Jurkat cells were incubated with NIH/3T3 fibroblasts transfected with either GFP alone (as control, not shown) or HA-tagged Dyn2WT (e) or Dyn2K44A (j) and the localization of FLAG-tagged Vps34 (f, k) on phagocytic cups containing apoptotic cells (g, l) was monitored. In Dyn2K44A transfected cells, recruitment of FLAG-Vps34 to the forming phagosome was disrupted (k, arrow), while Vps34 was efficiently recruited to the phagocytic cup in Dyn2WT (f) or GFP-transfected cells (not shown). Phalloidin-stained cells are included to better discriminate phagocytic cups (i, n), which are enriched in actin. Quantitation of these data are presented in (o).
(p) 293T cells were transiently transfected with the indicated proteins, lysed and immunoprecipitated using anti-Flag conjugated agarose beads and assessed by immunoblotting for the indicated proteins.
(q) GST-tagged Vps34 or ELMO1 proteins were expressed in bacteria and purified using GST-sepharose. Approximately 10 Îg purified His-Dyn2 was added to approximately 2 Îg of each GST-tagged protein. Protein interaction was assessed by immunoblotting.
(r) 293T cells were transiently transfected with the indicated proteins, lysed and immunoprecipitated using anti-GFP conjugated agarose beads and assessed by immunoblotting for the coprecipitating proteins. Rab5Q79L and Rab5S34N are considered to mimic for GTP and GDP-bound Rab5, respectively.