Figure 1. Organization of the Retina and the Known Visual Cycle.
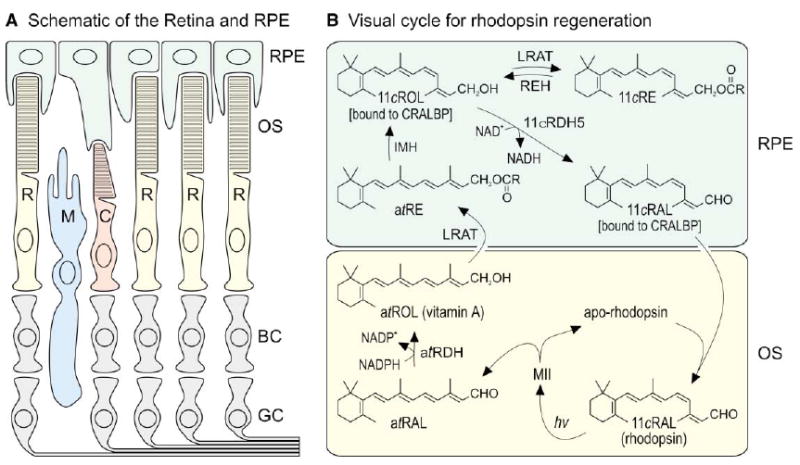
(A) Schematic drawing of a typical vertebrate retina. Rod (R) and cone (C) photoreceptors each contain an outer segment (OS), which is the site of photon capture and the reactions of visual transduction. Adjacent to the outer segments is the retinal pigment epithelium (RPE), which plays a role in the processing of retinoids but is not considered part of the retina. The Müller glial cell (M) may also play a role in retinoid processing. Second-order bipolar cells (BC) and third-order ganglion cells (GC) are shown but not discussed. (B) Visual cycle in rod-dominant retinas. Absorption of a single photon (hv) by a rhodopsin pigment molecule in the rod outer segment induces 11-cis to all-trans isomerization of the 11-cis-retinaldehyde (11cRAL) chromophore to form active metarhodopsin II (MII). MII rapidly decays to yield apo-rhodopsin and free all-trans-retinaldehyde (atRAL). The all-trans-retinaldehyde is reduced to all-trans-retinol (atROL) by all-trans-retinol dehydrogenase (atRDH), which uses NADPH as a cofactor (Lion et al., 1975; Palczewski et al., 1994; Rattner et al., 2000). The all-trans-retinol diffuses across the narrow extracellular space and is taken up by the RPE. Lecithin retinol acyl-transferase (LRAT) catalyzes trans-esterification of a fatty acid from phosphatidylcholine to all-trans-retinol, resulting in formation of an all-trans-retinyl ester (atRE) (Ruiz et al., 1999; Saari and Bredberg, 1989; Shi et al., 1993). Isomerohydrolase (IMH) is postulated to catalyze coupled hydrolysis and isomerization of all-trans-retinyl esters into 11-cis-retinol (11cROL) (Deigner et al., 1989). 11-cis-retinol is oxidized to 11-cis-retinaldehyde by 11-cis-retinol dehydrogenase type-5 (11cRDH5), which uses NAD+ as a cofactor (Lion et al., 1975; Simon et al., 1995). In the dark, under conditions of low 11-cis-retinaldehyde utilization, 11-cis-retinol can also be esterified by LRAT to form 11-cis-retinyl esters (11cRE). Cellular retinaldehyde binding protein (CRALBP) binds 11-cis-retinol and 11-cis-retinaldehyde with high affinity, but not all-trans-retinol (Bunt-Milam and Saari, 1983; Saari and Bredberg, 1987). With chromophore depletion, apo-CRALBP stimulates hydrolysis of 11-cis-retinyl esters by retinyl ester hydrolase (REH) (Stecher et al., 1999). 11-cis-retinaldehyde diffuses across the extracellular space, is taken up by the outer segment, and recombines with apo-rhodopsin to regenerate rhodopsin.
