Figure 6. 11-cis-Retinol Dehydrogenase Activity in Ground-Squirrel and Chicken Retinas.
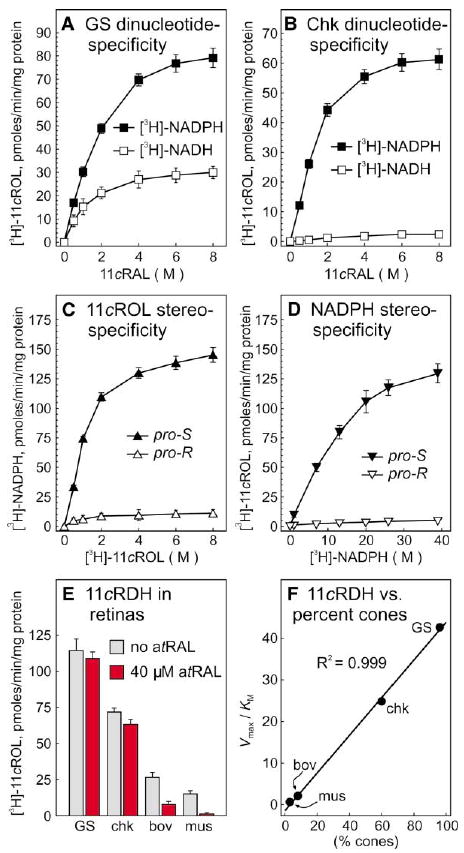
Microsomal membranes from ground squirrel (A) and chicken (B) retinas were assayed for the capacity to reduce 11-cis-retinaldehyde (11cRAL) to [3H]-11-cis-retinol (11cROL) in the presence of [3H]-NADH (open squares) or [3H]-NADPH (filled squares). 11-cis-retinol dehydrogenase (11cRDH) activity is expressed in pmols [3H]-11-cis-retinol per min per mg protein. (C) Ground-squirrel retinal microsomes were assayed for the capacity to synthesize [3H]-NADPH from pro-R- or pro-S-[3H]-11-cis-retinol plus unlabeled NADP+. (D) Ground-squirrel retinal microsomes were assayed for the capacity to synthesize [3H]-11-cis-retinol from pro-R- or pro-S-[3H]-NADPH. (E) Microsomes from ground squirrel (GS), chicken (chk), bovine (bov), and mouse (mus) retinas were assayed for the capacity to synthesize [3H]-11-cis-retinol from 11-cis-retinaldehyde and [3H]-NADPH in the absence (gray bars) or presence (red bars) of 40 μM all-trans-retinaldehyde (atRAL). Error bars show standard deviations (n = 4). (F) Derived Vmax/KM (catalytic efficiencies) for 11-cis-retinol dehydrogenase in mouse, bovine, chicken, and ground-squirrel retinas are plotted against the percentage of cone photoreceptors in each species. Linear regression analysis revealed a correlation coefficient (R2) of 0.999.
