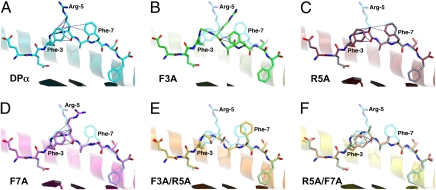
Fig. 1.
Peptide topology for the endogenous DPα and five APLs complexed with HLA-B*4402. In each panel, a cartoon representation is given of the Ag-binding cleft, whereas the bound peptide is presented in stick format. The α2-helix (residues 126–181) has been removed for clarity. In each of the APL panels, the index DPα peptide is superimposed in a semitransparent mode. Vdw interactions between atoms of P5 and other residues of the peptide are shown as blue dashes. (A) HLA-B*4402/DPα (1M6O; cyan carbon atoms). In addition to the interactions displayed, P5-Arg also interacts via its guanidinium head group with the side chain of Gln-155 (removed for clarity). (B) HLA-B*4402/DPα F3A (green carbon atoms). This APL displays an rmsd of 1.80 Å for all peptide atoms with respect to the index peptide and a maximum deviation of 3.27 Å in the side chain of P7-Phe. The side chain of P5-Arg is disordered beyond the Cγ atom. (C) HLA-B*4402/DPα R5A (red carbon atoms). Displayed is an rmsd of 0.24 Å for all peptide atoms with respect to the index epitope and a maximum deviation of 1.15 Å in the side chain of P8-Ser. (D) HLA-B*4402/DPα F7A (magenta). Rmsd for all peptide atoms is 1.06 Å. Maximum deviation is 5.61 Å in the side chain of P5-Arg. Interaction between P5-Arg and Gln-155 is maintained. (E) HLA-B*4402/DPα F3A/R5A (orange). All atom rmsd is 1.09 Å. Maximum deviation is 4.00 Å in the side chain of P5-Ala. (F) HLA-B*4402/DPα R5A/F7A (yellow). All atom rmsd is 0.47 Å. Maximum deviation is 1.59 Å in the side chain of P8-Ser.