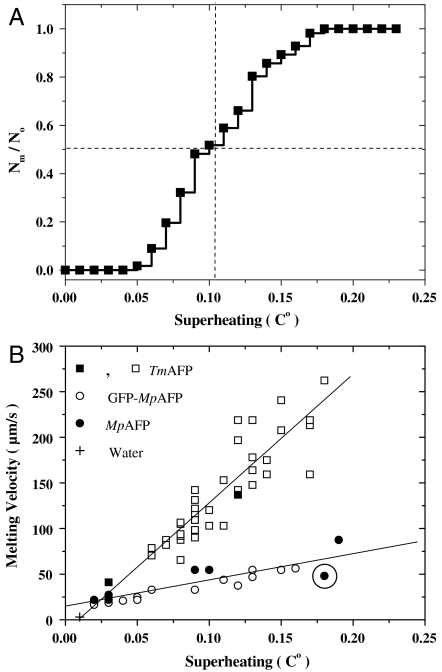
Fig. 3.
Analysis of melting nucleation and the melting velocities of ice crystals formed in AFP solutions. (A) Cumulative fraction of melted crystals as a function of superheating in a TmAFP solution. (B) Melting velocities of ice crystals stabilized in AFP solutions. Open squares correspond to the crystals in A. Solid squares represent data points from experiments using TmAFP solutions with different concentrations. Note that the melting velocities recorded in multiple crystal samples were comparable to those obtained using single crystal samples. Results of additional experiments with individual crystals in MpAFP (solid circles) and GFP-MpAFP (open circles) solutions are also shown. The circled data point corresponds to the experiment described in Fig. 1. The melting velocity of ice in pure water at 0.01 °C is marked with the symbol +.