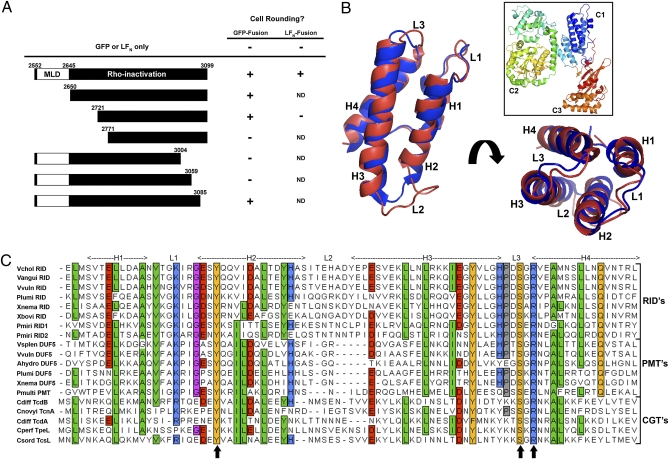
Fig. 1.
Deletion analysis of RIDVc identifies a broadly conserved domain. (A) Portions of the N- and C-termini of RIDVc were deleted from pRID-GFP or LFN-RID. Each truncated RIDVc was tested for its ability to cause cell rounding after transfection (GFP-fusions) or delivery to the cell with PA (LFN-fusions). (B) Cartoon of the entire PMT toxin (boxed, PDB ID: 2EBF) with the PMT C1 domain (blue) overlaid with a model of MLDVcRID (red). (C) Alignment of 19 putative MLDs from various bacterial toxins: see Table S1 for accession numbers and protein details. Shading shows aa conservation and residue description: green = hydrophobic; orange = polar; blue = basic; red = acidic. Arrows indicate aa mutated to either Ala or a similar residue on MLDVcRID or MLDVvDUF5.