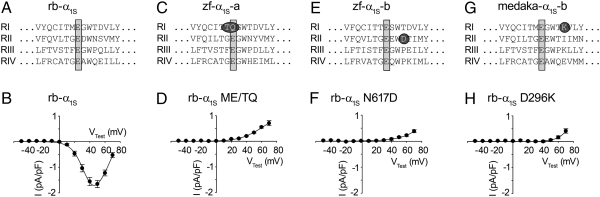
Fig. 4.
Amino acids incompatible with Ca2+ conductance. (A and B) In murine α1S-null myotubes, expression of the wild-type α1S from rabbit (rb-α1S), with a typical amino acid arrangement of a conducting channel in the pore region of homologous repeats I–IV (RI-RIV), could restore Ca2+ inward currents of 1.79 ± 0.16 pA/pF (n = 37). (C) In zf-α1S-a, a threonine and glutamine at position −1 and 0 relative to the selectivity filter (selectivity filter glutamates are indicated by gray bars) of homologous repeat I (RI) were identified and equally found in α1S-a of pike characin and medaka. (D) Corresponding replacement of residues Met291 and Glu292 to threonine and glutamine in rb-α1S (rb-α1S ME/TQ) completely abolished Ca2+ inward currents (n = 9). Similar results were obtained with (F) rb-α1S N617D (n = 8) in which Asn617 of rb-α1S was exchanged to aspartate as found in α1S-b of zebrafish (E) and pike characin, as well as with (H) rb-α1S D296K (n = 15) in which Asp296 was exchanged to lysine as found in α1S-b of medaka (G).