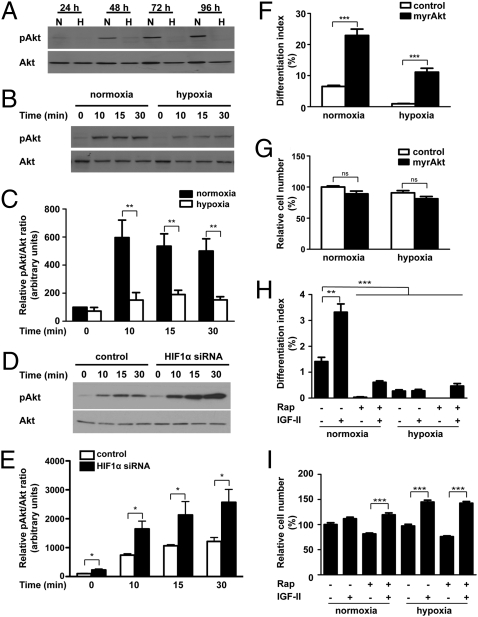
Fig. 3.
Hypoxia inhibits myogenic action of IGF-II by suppressing Akt-mTOR signaling through HIF-1–dependent mechanisms. (A) Hypoxia represses Akt signaling activity. C2C12 cells were induced to differentiate under normoxic or hypoxic conditions. Total and phosphorylated Akt levels were determined by Western blot at the time points indicated. (B and C) Hypoxia decreases IGF-induced Akt activation. After switching to DM for 72 h under normoxic or hypoxic conditions, IGF-I (50 ng/mL) was added. Cell lysates were harvested at the time points indicated and blotted for phospho- and total Akt (B). The phospho-Akt/total Akt ratio was quantified (C). Values are expressed as relative to that of the 0-h normoxia group. Data are mean ± SE, n = 6. (D and E) Effect of hypoxia on Akt activation is HIF-1-–dependent. C2C12 cells were transfected with pSUPER or pSUPER HIF-1α and switched to differentiation medium and incubated under hypoxia. After 72 h, cells were stimulated with IGF-I (50 ng/mL), and lysates were collected at different time points as indicated. Western blots were quantified (E). Values are expressed as relative to that of the 0-min control group. Data are mean ± SE, n = 4. (F and G) Expression of myrAkt restores myogenesis under hypoxia. C2C12 cells were transfected with control or myrAkt plasmid and induced to differentiate under normoxic or hypoxic conditions, and differentiation index (F) and cell number (G) were measured. Data are mean ± SE, n = 4. (H and I) Inhibition of mTOR activity by rapamycin decreases the myogenic action of IGF-II. Cells were induced to differentiate in the absence or presence of IGF-II (300 ng/mL) and/or rapamycin (200 nM) under normoxic or hypoxic conditions. After 36 h, cells were fixed and the differentiation index (H) and cell number (I) were determined. Data shown are means ± SE, n = 4–6.