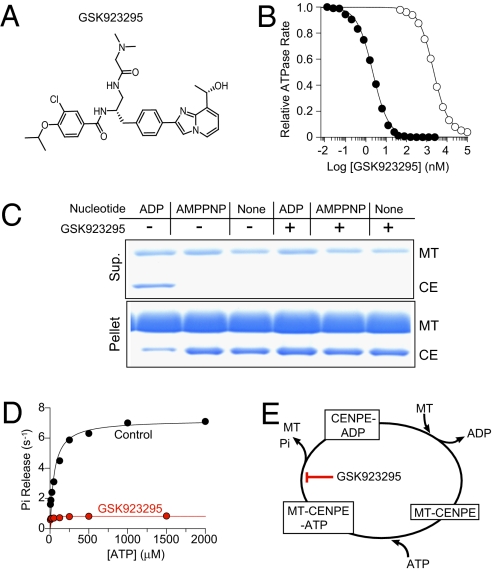
Fig. 1.
GSK923295 inhibits microtubule (MT)-stimulated ATPase of CENP-E and promotes formation of a MT-bound complex. (A) Chemical structure of GSK923295. (B) Concentration-dependent inhibition of CENP-E motor domain (1 nM) ATPase by GSK923295 in the presence (•) and absence (○) of 5 μM MT indicates a preference of GSK923295 for CENP-E-MT complex. GSK923295 inhibited CENP-E MT-stimulated ATPase with a Ki of 3.2 ± 0.2 nM in a manner uncompetitive with either MT or ATP (Table 1, Table S1, and Fig. S1). (C) Equilibrium binding and cosedimentation of CENP-E motor domain (CE; 3 μM) with MT (6 μM) in the presence or absence of the indicated nucleotides (1 mM) or in the presence or absence of 20 μM GSK923925 reveals inhibitor-induced loss of CENP-E motor domain from the supernatant and increase in the MT pellet, irrespective of nucleotide state. (Table S2). (D) Presteady-state rates of release of inorganic phosphate (Pi) from CENP-E motor domain in the presence (red) or absence (black) of GSK923295 as a function of ATP indicates that GSK923295 inhibits the production or release of Pi. (E) Summary model of the biochemical mechanism of action of GSK923295.